Review Article |
|
Corresponding author: Eduardo Ascarrunz ( eascarrunz@mailfence.com ) Corresponding author: Marcelo R. Sánchez-Villagra ( m.sanchez@pim.uzh.ch ) Academic editor: Ingmar Werneburg
© 2022 Eduardo Ascarrunz, Marcelo R. Sánchez-Villagra.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Ascarrunz E, Sánchez-Villagra MR (2022) The macroevolutionary and developmental evolution of the turtle carapacial scutes. Vertebrate Zoology 72: 29-46. https://doi.org/10.3897/vz.72.e76256
|
Abstract
The scutes of the carapace of extant turtles exhibit common elements in a narrow range of topographical arrangements. The typical arrangement has remained constant since its origin in the clade Mesochelydia (Early Jurassic), after a period of apparent greater diversity in the Triassic. This contribution is a review of the development and evolutionary history of the scute patterns of the carapace, seen through the lens of recent developmental models. This yields insights on pattern variations in the fossil record. We reinterpret the “supracaudal” scute and propose that Proganochelys had five vertebral scutes. We discuss the relationship between supramarginal scutes and Turing processes, and we show how a simple change during embryogenesis could account for origin of the configuration of the caudal region of the carapace in mesochelydians. We also discuss the nature of the decrease in number of scutes over the course of evolution, and whether macroevolutionary trends can be discerned. We argue that turtles with complete loss of scutes (e.g., softshells) follow clade-specific macroevolutionary regimes, which are distinct from the majority of other turtles. Finally, we draw a parallel between the variation of scute patterns on the carapace of turtles and the scale patterns in the pileus region (roof of the head) of squamates. The size and numbers of scales in the pileus region can evolve over a wide range, but we recognized tentative evidence of convergence towards a typical configuration when the scales become larger and fewer. Thus, typical patterns could be a more general property of similar systems of integumentary appendages.
Keywords
canalization, ontogeny, pholidosis, scales, Squamata, Testudines, variation
Introduction
A conspicuous feature of amniotes is the diversity of skin appendages that cover their bodies, such as hair in mammals, feathers in birds, and scales in reptiles (including the legs of birds). These appendages have been found to develop in the embryo from specialized plate-like patches of thickened epidermis, called placodes (Oliveira-Martinez et al. 2003;
Here, we review different aspects of an outstanding example of evolutionarily conserved pholidosis: the mosaic of scutes of the carapace of the dorsal shell of turtles (carapace). It is a rare opportunity to be able to study the evolutionary history of patterns of epidermal appendages in deep time with abundant palaeontological data, and the turtle shell probably provides the best material of this kind.
The first known turtles with a carapace (turtles with a carapace = clade Testudinata; crown turtles = clade Testudines) are from the Late Triassic (Norian, 227-208 Ma) (
In this contribution we integrate a series of recent studies in palaeontology and developmental biology, including a model based on reaction-diffusion processes (
We focus our discussion in the carapace, as the development of the plastron (ventral portion of the shell) has not been studied in similar detail. The developmental systems of scute patterning on the carapace and plastron are considered independent (
The extant turtle carapace and its scute arrangement
Both carapace and plastron are made up of an internal layer of bone plates with contributions of the ribs, and an external layer of scutes. Secondarily, the leatherback sea turtle Dermochelys coriacea, the soft-shelled turtles (Trionychidae) and Carettochelys insculpta do not have scales on the carapace. Soft-shelled turtles are particularly divergent in their shell and its mode of development (
Excepting the hawksbill sea turtle Eretmochelys imbricata, there is little or no overlap between scutes. The limits between adjacent scutes form epidermal furrows termed seams, following the terminology proposed by
The bone plates and scute mosaic of the carapace of the vast majority of modern turtles conform to a basic plan that appeared in the clade Mesochelydia (
Synthetic phylogeny of testudinatans highlighting many of the species and clades mentioned in the text. Based mainly on
The external anatomy of the bony carapace of testudinatans. Pholidosis is shown by the imprints (sulci) left by the borders of the corneous scutes. Kayentachelys aprix is the oldest known testudinatan that displays the complete mesochelydian plan: the general layout of bone plates and scutes that is preserved in the majority of living turtles, such as the emydid Malaclemys terrapin. Earlier testudinatans like Proganochelys quendstedti and Proterochersis porebensis (reconstructions) had more capacial scutes, and the series of marginal scutes did not meet in the posterior part of the carapace. Proterochersis also shows evidence of numerous irregular bone plates that are not present in mesochelydians; see Szczygielski & Sulej (2019) for details. Labelled elements are bone plates. Scute homologies are colour-coded. Thin lines represent bone plate sutures; thick lines represent sulci. The sutures in Proganochelys are unknown. Proganochelys after
The underlying bone plates follow a similar arrangement that is however non-congruent with the scute pattern (Fig.
Development of the scute mosaic
The following account synthesizes findings from
The major feature in the early development of the carapace are the “carapacial ridges”: two nearly parallel longitudinal bulges between the anterior and posterior limb buds, in the flanks of the dorsal region of the embryo (
In subsequent stages of turtle carapace development, a series of six pairs of placodes appears along the midline, on the dorsum of the trunk of the embryo. The five posterior pairs of placodes are the primordia of the five vertebral scutes, and the anterior pair are the primordia of the cervical scute (
The formation of the seams between the scutes has been described in detail by
The identities of all the carapacial scutes are thus settled, and their subsequent development is mostly concerned with changes in their proportions and further maturation of the epithelium (
Models of scute patterning
The turtle carapace provided one of the many examples that D’Arcy
Over the course of the last few decades, Cherepanov and colleagues (
A causal model to explain the generation of scute patterns based on Turing patterns was introduced by
The model of
This relatively simple model is highly successful in reproducing key features of the scute mosaic. The first reaction-diffusion process originating from the twelve pairs of marginal placodes induce the formation of two rows of four or five “pleural” placodes, and a medial row of six pairs of placodes representing the cervical and the five vertebrals. The second reaction-diffusion process mimics the growth of the primordia and the appearance of the scute seams. The result is most satisfactory in the middle region of the trunk, even reproducing the fusion of the pairs of primordia of vertebrals II, III, and IV (Fig.
Simulations of pholidosis of the carapace with a reaction-diffusion model. Darker colours indicate higher concentrations of activator of the first reaction diffusion-process (A1) or the inhibitor of the second reaction-diffusion process (I2). A, The beginning (t=0) and end (t=250000 iterations) of the simulation with the original parameters of
The model was also successful in replicating abnormal scute patterns of actual turtles (
The reaction-diffusion model provides a causal complement to the general thrust of the segment-dependent model. This is particularly relevant because, beyond the pre-pattern of twelve marginal placodes along the carapacial ridges, the reaction-diffusion model involves no concept of body segmentation. Still, it has not been explored to what extent the reaction-diffusion model can reproduce the range of intraspecific supernumerary scale variations that are strongly suggestive of the strict correspondence between myosepta and scute placodes (
Revisiting basal testudinatans
There are only eight known and undisputed species of non-mesochelydian turtles, most of them from the Norian (Late Triassic, 227–208.5 Ma; except Australochelys africanus from the Hettangian) (Fig.
The most complex pholidosis is seen in Proganochelys quendstedtii (Fig.
The almost complete scutation pattern can also be observed in Proterochersis porebensis and Proterochersis robusta, where there are typically one cervical, five vertebrals, four pairs of pleurals, 14 marginals (at least 12 in Proterochersis porebensis), and three supramarginals (
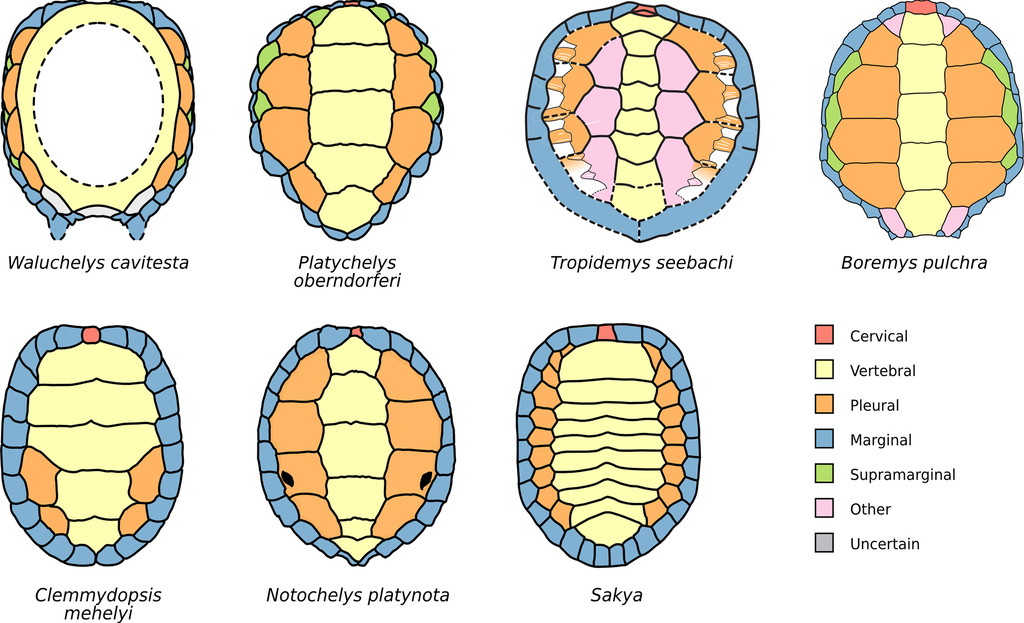
The left and right series of marginal scutes do not meet at the midline in the caudal region of the carapace in any of the earliest testudinatans where this condition can be ascertained: Proganochelys quendstedti, Proterochersis spp., Waluchelys cavitesta, and Palaeochersis talampayensis (
We propose, first, that the fundamental difference in the development of the caudal region of the carapace of basal testudinatans was the failure of the lateral marginal scute series to meet at the midline, posterior to the primordium of vertebral V. It is easy to derive this inference from the fact that in extant turtles the series of marginal scute primordia develop early along the carapacial ridges, when the ridges are roughly parallel to each other, on the flanks of the embryo. Thus, vertebral V remains at the postero-medial edge of the carapace, preserving the relative position of the scute primordia from the early stages of development. This leads us directly to a second proposition: that it might not be necessary to retain the notion of a distinct kind of scute called “supracaudal”, unique to basal testudinatans. Instead, we propose that the “supracaudal” attributed to Proganochelys and Waluchelys might be a very short vertebral V, with the same topological relations as seen in Proterochersis, except with respect to the supramarginals. Embryological observations and the reaction-diffusion model show that the all scutes along the midline of the carapace (the cervical and the vertebrals) have the same initial mode of development from paired placodes (
Thus, in our new interpretation, Proganochelys has five vertebral scutes, just as Proterochersis and the vast majority of mesochelydians. The pholidosis of the central region of the carapace of other basal testudinatans is unknown, and in the absence of contradictory evidence, it is reasonable to presume that they also had five vertebral scutes in total.
Another possible interpretation of the nature of the “supracaudal” of basal testudinatans, is that it is the result of the fusion of multiple marginal scutes, and therefore the marginal series meet in the caudal region of the carapace in Proganochelys and Waluchelys. A fusion of the XIIth pair of scutes occurs in tortoises (Testudinidae), which results in a single scute occupying the posteromedial edge of the carapace (see above) (
Comparison of the carapace pholidosis of several testudinatans. Waluchelys cavitesta after
Apart from the caudal notch, another distinctive feature of basal testudinatans is the presence of supramarginal scutes. This is the most difficult series of scutes to characterize, as their numbers and anatomical location vary widely across species. In addition to basal testudinatans, supramarginal scutes also occur in the alligator snapping turtle Macrochelys temminckii (
Macroevolutionary patterns, adaptations, and canalization
The evolution of the testudinatan shell from the Triassic to the present has resulted in a net reduction in the average number of its constituent elements. Various authors have thus recognized this as a macroevolutionary trend towards simplification of both the numbers of bony plates and epidermal scutes (
Here we take a closer look at the history of gains and losses of carapacial scutes. We will first consider the majority of turtles that retain the epidermal scutes. We will address turtles with complete loss of scutes separately.
The modern turtle shell displays a distinctive pholidotic pattern in that a large surface of the body is covered with a mosaic of few scales (typically 38 in the carapace; Fig.
Gains and losses of scutes. Minimal (most parsimonious) independently accumulated gains and losses of carapacial scutes apomorphic for selected species or clades of testudinatans. The “nuchal scute” (
| Clade or species | Cervicals | Vertebrals | Pleurals (pairs) | Marginals (pairs) | Other (pairs) | Reference |
| Mesochelydia | 0 | 0 | 0 | –2 to –4 | –2 to –12 |
|
| Elseya | –1 | 0 | 0 | 0 | 0 | Ascarrunz, unpublished |
| Kinosternidae | 0 | 0 | 0 | –1 | 0 |
|
| Lepidochelys olivacea (Carettinae) | 0 | 0 to +2 | +1 to +2 | +1 | 0 |
|
| Macrochelys | 0 | 0 | 0 | 0 | +3 to +4 |
|
| Notochelys platynota | 0 | +1 | 0 | 0 | 0 |
|
| Pelomedusoides | –1 | 0 | 0 | 0 | 0 | Ascarrunz, unpublished |
| Testudo graeca (Testudinidae) | –1 | 0 | 0 | –1 | 0 |
|
| Boremys grandis † (Eubaeninae) | +2 | +1 | 0 | 0? | +9? |
|
| Clemmydopsis mehelyi † | 0 | 0 | –2 | 0 | 0 |
|
| Kallokibotion † | –1 | 0 | 0 | 0 | 0 |
|
| Naomichelys speciosa † | 0 | 0 | 0 | 0 | +1 |
|
| Platychelys oberndorferi † | 0 | 0 | 0 | 0 | +3 |
|
| Pleurosternidae † | –1 | 0 | 0 | 0 | 0 |
|
| Sakya riabinini † | 0 | +5 | +6 | +2 | 0 |
|
| Tropidemys seebachi † (Thalassochelydia) | +1 | +3? | 0? | 0? | +3? |
|
| Total gains – losses | –2 | +10 to +12 | +5 to +6 | –1 to –3 | +7 to +18 |
Cordero & Vlachos (2021) presented the first quantitative analyses of the evolution of the numbers of shell elements with comparative methods. But they too acknowledge that scute gains and losses represent fairly rare events over the course of the evolutionary history of testudinatans, and that it is difficult to sample the relevant species in an unbiased manner. For this reason, the overall rate of change in scute number is bound to be small. If there are statistically identifiable trends in the dominant macroevolutionary regime, they are subtle. Properly characterizing them will require extensive scute number variation data, and a comprehensive time-scaled phylogeny of testudinatans. Neither are available at the moment.
Finally, we note that it is difficult to conceive a distinct and plausible mechanism for between-lineage downward trends in scute number. Between-lineage dynamics have been attributed to species selection (
It has been suggested that groups of species in a new “Bauplan” have more variability (i.e., more capacity to generate variation) than subsequent clades, which are less prone to vary (
The evolutionary history of testudinatan carapacial scutes involved different macroevolutionary regimes. We examine in the following subsections the clades that feature complete loss of shell scutes: Trionychia, and the marine turtles Dermochelyidae and Protostegidae. The developmental processes that produce total loss of scutes (see below) are quite different from the ones that produce the loss of individual scutes, where the total corneous coverage of the shell is preserved by fusions or compensatory expansions of other scutes in a space-filling manner (reviewed in
Macroevolutionary patterns and adaptations in Trionychia
The clade Trionychia includes the Trionychidae (softshell turtles) and Pan-Carettochelys (the scute-less pig-nosed turtle Carettochelys insculpta and numerous extinct relatives;
We infer that in early trionychids and the ancestors of Carettochelys, scute loss was driven by the adaptive value in the decornification of the skin covering the shell, and a relaxation of the strength of natural selection for the maintenance the developmental system of periodic patterning that determines the scute mosaics. Similar hypotheses have been put forward by previous authors (
Macroevolutionary patterns in sea turtles
It is more difficult to hypothesize about the drivers for scute loss in the marine turtle clades Protostegidae and Dermochelyidae. Only the latter is represented by an extant species, the leatherback turtle Dermochelys coriacea. The Protostegidae might be stem-chelonioids or the sister clade of Dermochelyidae (
Scales and developmental bias
It is illuminating to seek parallels between character systems in different clades. For the pholidosis of the turtle carapace, though, is difficult to come by with analogous (or homologous) character systems that display relevant similarities in the morphological, developmental, and evolutionary characteristics that we have discussed in this paper. For instance, the large scales on the body of pangolins do not grow in a surface-filling fashion forming a mosaic, as they are arranged in numerous overlapping rows (
We identified a more satisfactory analogue in a different anatomical region. There is great diversity in the pholidotic patterns of the head in squamates, including mosaics of scales of a wide range of sizes, from proper scutes to small “granular” scales. In squamates excluding gekkotans and dibamids, scutes are fairly common in the “pileus region”: the dorsal surface of the head extending from the tip of the rostrum to the occiput. Notably, when scutes are found in the pileus, they have a tendency to be form similar arrangements, and individual scutes can be recognized between species (
Pileus scalation patterns in squamates. Left: Traditionally hypothesized homologies of scales between different squamate clades. Identities become clear only when the scales are large, forming scutes. Diagrams after
In a manner roughly analogous to the mesochelydian plan, a stereotypical pileus scute pattern has been considered ancestral for squamates (
Based on photographic data collected for a previous study (
The head is a complex anatomical structure that tightly accommodates and supports diverse sense organs and mechanical feeding specializations, among other functions. Turtle shells, in contrast, provide a vessel for a large body cavity where organs are arranged more freely, and are routinely displaced due to head and limb tucking, food intake, and gravidity. Unlike bones, most specific scales are not intimately associated with other critical components of the head (except the interoccipital scale with the pineal eye in lizards, and scales surrounding eyes and nares), and therefore the topography of the scutes should be more prone to vary with respect to the geometric and mechanical diversity of the vertebrate head. A worthy avenue of research would be to examine if the evolution of pholidotic patterns and scale size reflect the parallel ecological adaptations seen in the gross head geometry of pythons and boas (
We infer that the scale systems of the turtle carapace and the pileus of squamates are subject to similar kinds of developmental biases (
In turtles, a release from the bias towards the mesochelydian plan occurs in trionychians and Dermochelys, where the lack of scutes obviates the need for preserving a patterning morphogenetic process (
Unfortunately, the fossil record is unlikely to shed light in the matter. Unlike the bony shell (
Conclusions
Developmental models aid palaeontologists to assess problems about character change in explicit causal frameworks. Even if the models do not suffice to explain all the relevant variation, they can shed light on matters previously only understood as far as the traditional approach of pattern-matching can reveal. In the carapace, the recent discoveries highlight the relationship between body segmentation and scute patterning, and how the same structures in the flanks of the carapace, –the carapacial ridges–, contribute to the formation of the outer ring of the carapace while possibly inducing its internal integumental patterning, as suggested by the temporal and causal priority of the marginals over the pleurals and vertebrals. We showed how the origin of the mesochelydian plan can be understood in these terms.
For the vast majority of turtles that retain their scutes, the macroevolutionary patterns are more complex and subtle than what might be conveyed by the “trend toward scute loss” that is traditionally suggested in the literature. Still, the reasons why scute number evolution remains highly constrained remain unknown, especially in contrast with what it is possible to induce in the developmental models, and also with what is seen in the diversity resulting from a similar developmental system in squamates.
On a more general note, we hope that this paper is an example of how considerations of ontogeny offer a deeper and necessary understanding of morphological transformations that occur in macroevolutionary time (
Acknowledgements
We are deeply indebted to Damien Esquerré for sharing his photographic data of boas and pythons. We thank Julien Claude, Serjoscha Evers, Torsten Scheyer, and Walter Joyce for discussions and bibliographic material, as well as Gerardo Cordero and an anonymous reviewer for fruitful suggestions and critiques. We thank Ingmar Werneburg and Irina Ruf for the chance to publish this work in honour of Wolfgang Maier and their leadership in this noble task.
MRSV thanks Roland Zimm and Isaac Salazar-Ciudad for the opportunity to participate in Roland’s doctoral thesis evaluation committee and thus become acquainted with this important and beautiful work.
MRSV expresses his deep gratitude to Wolfgang Maier for the exceptional professional opportunities he created to him and the mentorship over the many years, including of course countless discussions and teachings on comparative anatomy and developmental evolution.
References
- Alibardi L (2003) Adaptation to the land: The skin of reptiles in comparison to that of amphibians and endotherm amniotes. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 298B: 12–41. https://doi.org/10.1002/jez.b.24
- Alibardi L, Dipietrangelo L (2005) Differentiation of the epidermis of scutes in embryos and juveniles of the tortoise Testudo hermanni with emphasis on beta-keratinization. Acta Zoologica 86: 205–216. https://doi.org/10.1111/j.1463-6395.2005.00203.x
- Anquetin J, Joyce WG (2014) A reassessment of the Late Jurassic turtle Eurysternum wagleri (Eucryptodira, Eurysternidae). Journal of Vertebrate Paleontology 34: 1317–1328. https://doi.org/10.1080/02724634.2014.880449
- Ascarrunz E, Sánchez-Villagra MR, Betancur-R R, Laurin M (2019) On trends and patterns in macroevolution: Williston’s law and the branchiostegal series of extant and extinct osteichthyans. BMC Evolutionary Biology 19: 117. https://doi.org/10.1186/s12862-019-1436-x
- Biggs LC, Mikkola ML (2014) Early inductive events in ectodermal appendage morphogenesis. Seminars in Cell & Developmental Biology 25–26: 11–21. https://doi.org/10.1016/j.semcdb.2014.01.007
- Beggs K, Young J, Georges A, West P (2000) Ageing the eggs and embryos of the pig-nosed turtle, Carettochelys insculpta (Chelonia: Carettochelydidae), from northern Australia. Canadian Journal of Zoology 78: 373–392. https://doi.org/10.1139/z99-214
- Bentley BP, McGlashan JK, Bresette MJ, Wyneken J (2021) No evidence of selection against anomalous scute arrangements between juvenile and adult sea turtles in Florida. Journal of Morphology 282: 173–184. https://doi.org/10.1002/jmor.21294
- Benton MJ, Forth J, Langer MC (2014) Models for the rise of the dinosaurs. Current Biology 24: R87–R95. https://doi.org/10.1016/j.cub.2013.11.063
- Brophy TR, Ernst CH (2004) Sexual dimorphism, allometry and vertebral scute morphology in Notochelys platynota (Gray, 1834). Hamadryad 29: 80–88.
- Bujes CS, Verrastro L (2007) Supernumerary epidermal shields and carapace variation in Orbigny’s slider turtles, Trachemys dorbigni (Testudines, Emydidae). Revista Brasileira de Zoologia 24: 666–672. https://doi.org/10.1590/S0101-81752007000300018
- Burbrink FT, Grazziotin FG, Pyron RA, Cundall D, Donnellan S, Irish F, Keogh JS, Kraus F, Murphy RW, Noonan B, Raxworthy CJ, Ruane S, Lemmon AR, Lemmon EM, Zaher H (2020) Interrogating genomic-scale data for Squamata (Lizards, Snakes, and Amphisbaenians) shows no support for key traditional morphological relationships. Systematic Biology 69: 502–520. https://doi.org/10.1093/sysbio/syz062
- Burke AC (1989) Development of the turtle carapace: Implications for the evolution of a novel bauplan. Journal of Morphology 199: 363–378. https://doi.org/10.1002/jmor.1051990310
- Campbell JA, Frost DR (1993) Anguid lizards of the genus Abronia : revisionary notes, descriptions of four new species, a phylogenetic analysis, and key. Bulletin of the AMNH. Available from: http://digitallibrary.amnh.org/handle/2246/823 (February 27, 2020).
- Chen IH, Kiang JH, Correa V, Lopez MI, Chen P-Y, McKittrick J, Meyers MA (2011) Armadillo armor: mechanical testing and micro-structural evaluation. Journal of the Mechanical Behavior of Biomedical Materials 4: 713–722. https://doi.org/10.1016/j.jmbbm.2010.12.013
- Cherepanov G (2019) Morphogenetic and constructional differences of the carapace of aquatic and terrestrial turtles and their evolutionary significance. Journal of Morphology 280: 1571–1581. https://doi.org/10.1002/jmor.21050
- Cherepanov GO (1992) New morphogenetic data on the turtle shell: discussion on the origin of the horny and bony parts. Studia Geologica Salmanticensia. Studia Geologica Salmanticensia. Studia Palaeocheloniologica 3: 9–24.
- Cherepanov GO (2006) Ontogenesis and evolution of horny parts of the turtle shell. In: Danilov IG, Parham JF (Eds) , Fossil turtle research. Russian Journal of Herpetology. Saint Petersburg, Russia, 19–33.
- Cherepanov GO (2014) Patterns of scute development in turtle shell: Symmetry and asymmetry. Paleontological Journal 48: 1275–1283. https://doi.org/10.1134/S0031030114120028
- Cherepanov GO (2015) Scute’s polymorphism as a source of evolutionary development of the turtle shell. Paleontological Journal 49: 1635–1644. https://doi.org/10.1134/S003103011514004X
- Cherepanov GO (2016) Nature of the turtle shell: morphogenetic causes of bone variability and its evolutionary implication. Paleontological Journal 50: 1641–1648. http://dx.doi.org/10.1134/S0031030116140033
- Cherepanov GO, Malashichev Y, Danilov I (2019) Supernumerary scutes verify a segment-dependent model of the horny shell development in turtles. Journal of Anatomy 235: 836–846. https://doi.org/10.1111/joa.13022
- Clarac F, Scheyer TM, Desojo JB, Cerda IA, Sanchez S (2020) The evolution of dermal shield vascularization in Testudinata and Pseudosuchia: phylogenetic constraints versus ecophysiological adaptations. Philosophical Transactions of the Royal Society B: Biological Sciences 375: 20190132. https://doi.org/10.1098/rstb.2019.0132
- Cope ED (1886) An analytical table of the genera of snakes. Proceedings of the American Philosophical Society 23: 479–499.
- Cope ED (1898) The crocodilians, lizards, and snakes of North America. In: Report of the United States National Museum for the year ending June 30, 1898. Government Printing Office, Washington DC, United States, 153–270. Available from: http://repository.si.edu/xmlui/handle/10088/29934 (August 21, 2021).
- Cordero GA (2021) Disentangling the correlated evolution of body size, life history, and ontogeny in miniaturized chelydroid turtles. Evolution & Development 23: 439–458. https://doi.org/10.1111/ede.12386
- Cordero GA, Vlachos E (2021) Reduction, reorganization and stasis in the evolution of turtle shell elements. Biological Journal of the Linnean Society. https://doi.org/10.1093/biolinnean/blab122
- Danilov IG (2005) Die fossilen Schildkröten Europas. In: Fritz U (Ed.) , Handbuch der Reptilien und Amphibien Europas. Band 3/IIIB: Schildkröten (Testudines) II (Cheloniidae, Dermochelyidae, Fossile Schildkröten Europas). AULA-Verlag, Wiebelsheim, Germany, 329–441.
- Davenport J, Plot V, Georges J-Y, Doyle TK, James MC (2011) Pleated turtle escapes the box – shape changes in Dermochelys coriacea. Journal of Experimental Biology 214: 3474–3479. https://doi.org/10.1242/jeb.057182
- Delfino M, Scheyer TM, Fritz U, Sánchez-Villagra MR (2010) An integrative approach to examining a homology question: shell structures in soft-shell turtles. Biological Journal of the Linnean Society 99: 462–476. https://doi.org/10.1111/j.1095-8312.2009.01356.x
- Di-Poï N, Milinkovitch MC (2016) The anatomical placode in reptile scale morphogenesis indicates shared ancestry among skin appendages in amniotes. Science Advances 2: e1600708. https://doi.org/10.1126/sciadv.1600708
- Dhouailly D, Godefroit P, Martin T, Nonchev S, Caraguel F, Oftedal O (2019) Getting to the root of scales, feather and hair: As deep as odontodes? Experimental Dermatology 28: 503–508. https://doi.org/10.1111/exd.13391
- Dunson WA (1986) Estuarine Populations of the snapping turtle (Chelydra) as a model for the evolution of marine adaptations in reptiles. Copeia 1986: 741–756. https://doi.org/10.2307/1444958
- Erwin DH (2007) Disparity: morphological pattern and developmental context. Palaeontology 50: 57–73. https://doi.org/10.1111/j.1475-4983.2006.00614.x
- Escalona T, Weadick CJ, Antunes A (2017) Adaptive patterns of mitogenome evolution are associated with the loss of shell scutes in turtles. Molecular Biology and Evolution 34: 2522–2536. https://doi.org/10.1093/molbev/msx167
- Esquerré D, Keogh JS (2016) Parallel selective pressures drive convergent diversification of phenotypes in pythons and boas. Ecology Letters 19: 800–809. https://doi.org/10.1111/ele.12620
- Estes R, de Queiroz K, Gauthier J (1988) Phylogenetic relationships within Squamata. In: Estes R, Pregill G (Eds) , Phylogenetic relationships of the lizard families. Stanford University Press, Stanford, California, United States, 119–281.
- Evers SW, Benson RBJ (2019) A new phylogenetic hypothesis of turtles with implications for the timing and number of evolutionary transitions to marine lifestyles in the group. Palaeontology 62: 93–134. https://doi.org/10.1111/pala.12384
- Evers SW, Barrett PM, Benson RBJ (2019a) Anatomy of Rhinochelys pulchriceps (Protostegidae) and marine adaptation during the early evolution of chelonioids. PeerJ 7: e6811. https://doi.org/10.7717/peerj.6811
- Evers SW, Neenan JM, Ferreira GS, Werneburg I, Barrett PM, Benson RBJ (2019b) Neurovascular anatomy of the protostegid turtle Rhinochelys pulchriceps and comparisons of membranous and endosseous labyrinth shape in an extant turtle. Zoological Journal of the Linnean Society: zlz063. https://doi.org/10.1093/zoolinnean/zlz063
- Evers SW, Rollot Y, Joyce WG (2021) New interpretation of the cranial osteology of the Early Cretaceous turtle Arundelemys dardeni (Paracryptodira) based on a CT-based re-evaluation of the holotype. PeerJ 9: e11495. https://doi.org/10.7717/peerj.11495
- Flatt T (2005) The evolutionary genetics of canalization. The Quarterly Review of Biology 80: 287–316. https://doi.org/10.1086/432265
- Fofonjka A, Milinkovitch MC (2021) Reaction-diffusion in a growing 3D domain of skin scales generates a discrete cellular automaton. Nature Communications 12: 2433. https://doi.org/10.1038/s41467-021-22525-1
- Fossette S, Gleiss AC, Myers AE, Garner S, Liebsch N, Whitney NM, Hays GC, Wilson RP, Lutcavage ME (2010) Behaviour and buoyancy regulation in the deepest-diving reptile: the leatherback turtle. Journal of Experimental Biology 213: 4074–4083. https://doi.org/10.1242/jeb.048207
- Gadow H (1899) Orthogenetic variation in the shells of Chelonia. Arthur Willey’s Zoological Results, part 3: 207–222.
- Gaffney ES (1972) The systematics of the North American family Baenidae (Reptilia, Cryptodira). Bulletin of the AMNH ; v. 147, article 5. Available from: https://digitallibrary.amnh.org/handle/2246/1098 (May 26, 2021).
- Gaffney ES (1990) The comparative osteology of the Triassic turtle Proganochelys. Bulletin of the American Museum of Natural History 194: 1–263.
- Gaffney ES, Hutchison JH, Jenkins FA, Meeker LJ (1987) Modern turtle origins: the oldest known cryptodire. Science 237: 289–291. https://doi.org/10.1126/science.237.4812.289
- Gauthier J, Kearney M, Bezy RL (2008) Homology of cephalic scales in xantusiid lizards, with comments on night lizard phylogeny and morphological evolution. Journal of Herpetology 42: 708. https://doi.org/10.1670/07-047R2.1
- Georgalis GL, Joyce WG (2017) A review of the fossil record of Old World turtles of the clade Pan-Trionychidae. Bulletin of the Peabody Museum of Natural History 58: 115–208. https://doi.org/10.3374/014.058.0106
- Georgalis GL, Smith KT (2020) Constrictores Oppel, 1811 – the available name for the taxonomic group uniting boas and pythons. Vertebrate Zoology 70: 291–304. https://doi.org/10.26049/VZ70-3-2020-03
- Gierer A, Meinhardt H (1972) A theory of biological pattern formation. Kybernetik 12: 30–39. https://doi.org/10.1007/BF00289234
- Gilbert SF, Loredo GA, Brukman A, Burke AC (2001) Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evolution & development 3: 47–58.
- Girgis S (1961) Aquatic respiration in the common nile turtle Trionyx triunguis (Forskål). Comparative Biochemistry and Physiology 3: 206–217. https://doi.org/10.1016/0010-406X(61)90056-1
- Guerrero A, Pérez-García A (2021) Morphological variability and shell characterization of the European uppermost Jurassic to lowermost Cretaceous stem turtle Pleurosternon bullockii (Paracryptodira, Pleurosternidae). Cretaceous Research 125: 104872. https://doi.org/10.1016/j.cretres.2021.104872
- Harmon LJ (2018) Phylogenetic comparative methods: learning from trees. Available from: https://lukejharmon.github.io/pcm (May 22, 2018).
- Harvey MG, Rabosky DL (2018) Continuous traits and speciation rates: alternatives to state-dependent diversification models. Methods in Ecology and Evolution 9: 984–993. https://doi.org/10.1111/2041-210X.12949
- Herrera-Alsina L, van Els P, Etienne RS (2019) Detecting the dependence of diversification on multiple traits from phylogenetic trees and trait data. Systematic Biology 68: 317–328. https://doi.org/10.1093/sysbio/syy057
- Hirayama R (1997) Distribution and diversity of Cretaceous chelonioids. In: Callaway J, Nicholls EL (Eds) , Ancient marine reptiles. Academic Press, 225–241.
- Hirayama R, Chitoku T (1996) 1022 Family Dermochelyidae (superfamily Chelonioidea) from the Upper Cretaceous of North Japan. In: Transactions and proceedings of the Paleontological Society of Japan. New series. Palaeontological Society of Japan, 597–622.
- Horváth E, Danko S, Havaš P, Schindler M, Šebela M, Halpern B, Csibrány B, Farkas B, Kaňuch P, Uhrin M (2021) Variation in shell morphology of the European pond turtle, Emys orbicularis, in fragmented central European populations. Biological Journal of the Linnean Society 132: 134–147. https://doi.org/10.1093/biolinnean/blaa184
- Hunt G (2006) Fitting and comparing models of phyletic evolution: random walks and beyond. Paleobiology 32: 578–601. https://doi.org/10.1666/05070.1
- Jablonski D (2008) Species selection: theory and data. Annual Review of Ecology, Evolution, and Systematics 39: 501–524. https://doi.org/10.1146/annurev.ecolsys.39.110707.173510
- Joyce WG (2014) A review of the fossil record of turtles of the clade Pan-Carettochelys. Bulletin of the Peabody Museum of Natural History 55: 3–33.
- Joyce WG (2016) A review of the fossil record of turtles of the clade Pan-Chelydridae. Bulletin of the Peabody Museum of Natural History 57: 21–56.
- Joyce WG (2017) A review of the fossil record of basal Mesozoic Turtles. Bulletin of the Peabody Museum of Natural History 58: 65–113. https://doi.org/10.3374/014.058.0105
- Joyce WG, Bell CJ (2004) A review of the comparative morphology of extant testudinoid turtles (Reptilia: Testudines). Asiatic Herpetological Research 10: 53–109.
- Joyce WG, Lucas SG, Scheyer TM, Heckert AB, Hunt AP (2009) A thin-shelled reptile from the Late Triassic of North America and the origin of the turtle shell. Proceedings of the Royal Society B: Biological Sciences 276: 507–513. https://doi.org/10.1098/rspb.2008.1196
- Joyce WG, Lyson TR (2015) A Review of the fossil record of turtles of the clade Baenidae. Bulletin of the Peabody Museum of Natural History 56: 147–183. https://doi.org/10.3374/014.056.0203
- Joyce WG, Bourque JR (2016) A review of the fossil record of turtles of the clade Pan-Kinosternoidea. Bulletin of the Peabody Museum of Natural History 57: 57–95.
- Joyce WG, Mäuser M (2020) New material of named fossil turtles from the Late Jurassic (late Kimmeridgian) of Wattendorf, Germany. PLOS ONE 15: e0233483. https://doi.org/10.1371/journal.pone.0233483
- Joyce WG, Sterli J, Chapman SD (2014) The skeletal morphology of the solemydid turtle Naomichelys speciosa from the Early Cretaceous of Texas. Journal of Paleontology 88: 1257–1287. https://doi.org/10.1666/14-002
- Joyce WG, Micklich N, Schaal SFK, Scheyer TM (2012) Caught in the act: the first record of copulating fossil vertebrates. Biology Letters 8: 846–848. https://doi.org/10.1098/rsbl.2012.0361
- Kordikova EG (2002) Heterochrony in the evolution of the shell of Chelonia. Part 1: Terminology, Cheloniidae, Dermochelyidae, Trionychidae, Cyclanorbidae and Carettochelyidae. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen: 343–417. https://doi.org/10.1127/njgpa/226/2002/343
- Li C, Fraser NC, Rieppel O, Wu X-C (2018) A Triassic stem turtle with an edentulous beak. Nature 560: 476–479. https://doi.org/10.1038/s41586-018-0419-1
- Li C, Wu X-C, Rieppel O, Wang L-T, Zhao L-J (2008) An ancestral turtle from the Late Triassic of southwestern China. Nature 456: 497–501. https://doi.org/10.1038/nature07533
- Lichtig AJ, Lucas SG (2021) Chinlechelys from the Upper Triassic of New Mexico, USA, and the origin of turtles. Palaeontologia Electronica 24: a13. https://doi.org/10.26879/886
- Lively JR (2016) Baenid turtles of the Kaiparowits Formation (Upper Cretaceous: Campanian) of southern Utah, USA. Journal of Systematic Palaeontology 14: 891–918. https://doi.org/10.1080/14772019.2015.1120788
- Lyson TR, Bever GS, Scheyer TM, Hsiang AY, Gauthier JA (2013) Evolutionary origin of the turtle shell. Current Biology 23: 1113–1119. https://doi.org/10.1016/j.cub.2013.05.003
- Maddison WP, Midford PE, Otto SP (2007) Estimating a binary character’s effect on speciation and extinction. Systematic Biology 56: 701–710. https://doi.org/10.1080/10635150701607033
- Maffucci F, Pace A, Affuso A, Ciampa M, Treglia G, Pignalosa A, Hochscheid S (2020) Carapace scute pattern anomalies in the loggerhead turtle: are they indicative of hatchling’s survival probability? Journal of Zoology 310: 315–322. https://doi.org/10.1111/jzo.12754
- Maier W (2021) Der Weg zum Menschen. 2nd Edition. Scidinge Hall
- Milinkovitch MC, Manukyan L, Debry A, Di-Poï N, Martin S, Singh D, Lambert D, Zwicker M (2013) Crocodile head scales are not developmental units but emerge from physical cracking. Science 339: 78–81. https://doi.org/10.1126/science.1226265
- Moustakas-Verho JE, Cherepanov GO (2015) The integumental appendages of the turtle shell: An evo-devo perspective. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 324: 221–229. https://doi.org/10.1002/jez.b.22619
- Moustakas-Verho JE, Zimm R, Cebra-Thomas J, Lempiäinen NK, Kallonen A, Mitchell KL, Hämäläinen K, Salazar-Ciudad I, Jernvall J, Gilbert SF (2014) The origin and loss of periodic patterning in the turtle shell. Development 141: 3033–3039. https://doi.org/10.1242/dev.109041
- Nagashima H, Kuraku S, Uchida K, Kawashima-Ohya Y, Narita Y, Kuratani S (2012) Body plan of turtles: an anatomical, developmental and evolutionary perspective. Anatomical science international 87: 1–13.
- Nagashima H, Shibata M, Taniguchi M, Ueno S, Kamezaki N, Sato N (2014) Comparative study of the shell development of hard- and soft-shelled turtles. Journal of Anatomy 225: 60–70. https://doi.org/10.1111/joa.12189
- Nakajima Y, Danilov IG, Hirayama R, Sonoda T, Scheyer TM (2017) Morphological and histological evidence for the oldest known softshell turtles from Japan. Journal of Vertebrate Paleontology 37: e1278606. https://doi.org/10.1080/02724634.2017.1278606
- Nakov T, Beaulieu JM, Alverson AJ (2019) Diatoms diversify and turn over faster in freshwater than marine environments. Evolution 73: 2497–2511. https://doi.org/10.1111/evo.13832
- Newman HH (1906) The significance of scute and plate “abnormalities” in Chelonia: a contribution to the evolutionary history of the chelonian carapace and plastron. The Biological Bulletin 10: 68–107.
- Olivera-Martinez I, Viallet JP, Michon F, Pearton DJ, Dhouailly D (2004) The different steps of skin formation in vertebrates. The International Journal of Developmental Biology 48: 107–115. https://doi.org/10.1387/ijdb.15272376
- Pérez-García A, Codrea V (2018) New insights on the anatomy and systematics of Kallokibotion Nopcsa, 1923, the enigmatic uppermost Cretaceous basal turtle (stem Testudines) from Transylvania. Zoological Journal of the Linnean Society 182: 419–443. https://doi.org/10.1093/zoolinnean/zlx037
- Pimiento C, Tang KL, Zamora S, Klug C, Sánchez-Villagra MR (2018) Assessing canalisation of intraspecific variation on a macroevolutionary scale: the case of crinoid arms through the Phanerozoic. PeerJ 6: e4899. https://doi.org/10.7717/peerj.4899
- Pritchard PCH (1979) Encyclopedia of turtles. 1st ed. TFH New Jersey, United States.
- Procter JB (1922) A study of the remarkable tortoise, Testudo loveridgii Blgr., and the morphogeny of the chelonian carapace. Proceedings of the Zoological Society of London 92: 483–526. https://doi.org/10.1111/j.1096-3642.1922.tb02155.x
- Reynolds RG, Niemiller ML, Revell LJ (2014) Toward a tree-of-life for the boas and pythons: multilocus species-level phylogeny with unprecedented taxon sampling. Molecular Phylogenetics and Evolution 71: 201–213. https://doi.org/10.1016/j.ympev.2013.11.011
- Rice R, Riccio P, Gilbert SF, Cebra-Thomas J (2015) Emerging from the rib: resolving the turtle controversies. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 324: 208–220. https://doi.org/10.1002/jez.b.22600
- Rieppel O (2017) Turtles as hopeful monsters: origins and evolution. Indiana University Press, Bloomington, Indiana, United States, 208 pp.
- Rougier GW, de la Fuente MS, Arcucci AB (1995) Late Triassic turtles from South America. Science 268: 855–858.
- Sánchez-Villagra MR, Müller H, Sheil CA, Scheyer TM, Nagashima H, Kuratani S (2009) Skeletal development in the Chinese soft-shelled turtle Pelodiscus sinensis (Testudines: Trionychidae). Journal of Morphology 270: 1381–1399. https://doi.org/10.1002/jmor.10766
- Scheyer T, Martin Sander P, Joyce W, Bohme W, Witzel U (2007) A plywood structure in the shell of fossil and living soft-shelled turtles (Trionychidae) and its evolutionary implications. Organisms Diversity & Evolution 7: 136–144. http://dx.doi.org/10.1016/j.ode.2006.03.002
- Scheyer TM, Brüllmann B, Sánchez-Villagra MR (2008) The ontogeny of the shell in side-necked turtles, with emphasis on the homologies of costal and neural bones. Journal of Morphology 269: 1008–1021. https://doi.org/10.1002/jmor.10637
- Scheyer TM, Werneburg I, Mitgutsch C, Delfino M, Sánchez-Villagra MR (2013) Three ways to tackle the turtle: integrating fossils, comparative embryology, and microanatomy. In: Brinkman DB, Holroyd PA, Gardner JD (Eds) , Morphology and Evolution of Turtles. Vertebrate Paleobiology and Paleoanthropology. Springer Netherlands, Dordrecht, 63–70. https://doi.org/10.1007/978-94-007-4309-0_6
- Schoch RR, Sues H-D (2015) A Middle Triassic stem-turtle and the evolution of the turtle body plan. Nature 523: 584–587. https://doi.org/10.1038/nature14472
- Scholes NS, Schnoerr D, Isalan M, Stumpf MPH (2019) A Comprehensive network atlas reveals that Turing patterns are common but not robust. Cell Systems 9: 243–257.e4. https://doi.org/10.1016/j.cels.2019.07.007
- Simpson GG, Williams CS (1938) Crossochelys, Eocene horned turtle from Patagonia. Bulletin of the American Museum of Natural History 74: 221–254.
- Sterli J, Fuente MS de la (2013) New evidence from the Palaeocene of Patagonia (Argentina) on the evolution and palaeo-biogeography of Meiolaniformes (Testudinata, new taxon name). Journal of Systematic Palaeontology 11: 835–852. https://doi.org/10.1080/14772019.2012.708674
- Sterli J, Martínez RN, Cerda IA, Apaldetti C (2021) Appearances can be deceptive: bizarre shell microanatomy and histology in a new Triassic turtle (Testudinata) from Argentina at the dawn of turtles. Papers in Palaeontology 7: 1097–1132. https://doi.org/10.1002/spp2.1334
- Stone PA, Dobie JL, Henry RP (1992) Cutaneous surface area and bimodal respiration in soft-shelled (Trionyx spiniferus), stinkpot (Sternotherus odoratus), and mud turtles (Kinosternon subrubrum). Physiological Zoology 65: 311–330.
- Sullivan PM, Joyce WG (2017) The shell and pelvic anatomy of the Late Jurassic turtle Platychelys oberndorferi based on material from Solothurn, Switzerland. Swiss Journal of Palaeontology 136: 323–343. https://doi.org/10.1007/s13358-017-0136-7
- Szczygielski T, Sulej T (2016) Revision of the Triassic European turtles Proterochersis and Murrhardtia (Reptilia, Testudinata, Proterochersidae), with the description of new taxa from Poland and Germany. Zoological Journal of the Linnean Society 177: 395–427. https://doi.org/10.1111/zoj.12374
- Szczygielski T, Sulej T (2019) The early composition and evolution of the turtle shell (Reptilia, Testudinata). Benson R (Ed.). Palaeontology 62: 375–415. https://doi.org/10.1111/pala.12403
- Szczygielski T, Słowiak J, Dróżdż D (2018) Shell variability in the stem turtles Proterochersis spp. PeerJ 6: e6134. https://doi.org/10.7717/peerj.6134
- Thompson DW (1942) On growth and form. 2nd ed. Cambridge University Press, London.
- Thomson RC, Spinks PQ, Shaffer HB (2021) A global phylogeny of turtles reveals a burst of climate-associated diversification on continental margins. Proceedings of the National Academy of Sciences 118. https://doi.org/10.1073/pnas.2012215118
- Tong H, Brinkman D (2013) A new species of Sinemys (Testudines: Cryptodira: Sinemydidae) from the Early Cretaceous of Inner Mongolia, China. Palaeobiodiversity and Palaeoenvironments 93: 355–366.
- Turing AM (1952) The chemical basis of morphogenesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 237: 37–72.
- Turtle Taxonomy Working Group (2017) Turtles of the world: Annotated checklist and atlas of taxonomy, synonymy, distribution, and conservation status (8th Ed.). Chelonian Research Monographs 7: 1–292. https://doi.org/10.3854/crm.7.checklist.atlas.v8.2017
- Uller T, Moczek AP, Watson RA, Brakefield PM, Laland KN (2018) Developmental bias and evolution: a regulatory network perspective. Genetics 209: 949–966.
- Ultsch GR, Herbert CV, Jackson DC (1984) The comparative physiology of diving in North American freshwater turtles. I. Submergence tolerance, gas exchange, and acid-base balance. Physiological Zoology 57: 620–631.
- Ursel F (1978) Der Pileus der Squamata. Stuttgarter Beiträge zur Naturkunde 307. Available from: https://www.biodiversitylibrary.org/page/33430927.
- Waddington CH (1957) The strategy of the genes. Routledge, London, 274 pp.
- Wang B, Yang W, Sherman VR, Meyers MA (2016) Pangolin armor: overlapping, structure, and mechanical properties of the keratinous scales. Acta Biomaterialia 41: 60–74. https://doi.org/10.1016/j.actbio.2016.05.028
- Weinell J, Hooper E, Leviton A, Brown R (2019) Illustrated key to the snakes of the Philippines. Proceedings of the California Academy of Sciences 66: 1–49.
- Werneburg I, Maier W (2019) Diverging development of akinetic skulls in cryptodire and pleurodire turtles: an ontogenetic and phylogenetic study. Vertebrate Zoology 69 (2), 113–143.
- Werner F (1899) Phylogenetische Studien über die Homologien und Veränderungen der Kopfschilder bei den Schlangen. Arbeiten aus dem Zoologischen Institute der Universität Wien und der Zoologischen Station in Triest 11: 117–162.
- Yntema CL (1968) A series of stages in the embryonic development of Chelydra serpentina. Journal of Morphology 125: 219–251. https://doi.org/10.1002/jmor.1051250207
- Zacharias HCE (1898) Die Phylogenese der Kopfschilder bei den Boiden. 35 pp. Available from: http://archive.org/details/biostor-181221 (August 12, 2021).
- Zangerl R (1959) Rudimentäre Carapaxbeschuppung bei jungen Exemplaren von Carettochelys und ihre morphogenetische Bedeutung. Vierteljahrsschrift der Naturforschenden Gesellschaft in Zürich: 10.
- Zangerl R (1960) 3 The vertebrate fauna of the Selma Formation of Alabama. Part V. An advanced cheloniid sea turtle. Chicago Natural History Museum, Chicago, Illinois, United States, 60 pp. Available from: https://www.biodiversitylibrary.org/item/25178.
- Zangerl R (1969) The turtle shell. In: Gans C, Bellairs A d’Albini, Parsons TS (Eds) , Biology of the Reptilia. Morphology A. Academic Press, London and New York, 311–339.
- Zangerl R, Johnson RG (1957) The nature of shield abnormalities in the turtle shell. [Chicago]: Chicago Natural History Museum, 36 pp. Available from: http://archive.org/details/natureofshieldab1029zang (July 1, 2021).
- Zimm R (2019) On the development of the turtle scute pattern and the origins of its variation. PhD thesis. University of Helsinki Available from: https://helda.helsinki.fi/handle/10138/299142 (May 21, 2021).
- Zimm R, Bentley BP, Wyneken J, Moustakas-Verho JE (2017) Environmental causation of turtle scute anomalies in ovo and in silico. Integrative and Comparative Biology 57: 1303–1311. https://doi.org/10.1093/icb/icx066
Supplementary material
Control files for simulations
Data type: .zip
Explanation note: Control files for simulations with the reaction-diffusion model.