Research Article |
|
Corresponding author: Kole M. Kubicek ( kolekubicek@gmail.com ) Academic editor: Ralf Britz
© 2022 Kole M. Kubicek.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Kubicek KM (2022) Developmental osteology of Ictalurus punctatus and Noturus gyrinus (Siluriformes: Ictaluridae) with a discussion of siluriform bone homologies. Vertebrate Zoology 72: 661-727. https://doi.org/10.3897/vz.72.e85144
|
Abstract
The skeleton of Siluriformes is characterized by several autapomorphies, including secondary absence, extreme modification, and purported fusion of several ossifications. Although well documented in adults, information on skeletal development in catfishes is relatively sparse and typically focused on particular regions of the skeleton (e.g., Weberian apparatus). To further our understanding of the siluriform skeleton, I document the development of the entire skeleton in two ictalurid species, Ictalurus punctatus (channel catfish) and Noturus gyrinus (tadpole madtom) from five days pre-hatch to adult. I reexamine the homologies of bones previously hypothesized to represent compound elements in catfishes as well as an additional element only known to occur in some ictalurids. Development of the skeleton is complete in I. punctatus at 22.4 mm SL and almost complete in N. gyrinus (except dorsal- and anal-fin distal radials) at 14.1 mm SL. No signs of ontogenetic fusion were observed in any of the purported compound elements. Previous hypotheses of the homology of these elements and of additional ossifications are reviewed in light of developmental information obtained herein. No dermal parietal component is present at any stage in the so-called parieto-supraoccipital. The bone is the supraoccipital which ossifies from two lateral centers of ossification which later fuse, rather than from a median center. The ‘posttemporo-supracleithrum’ originates from a single center of ossification and represents the supracleithrum. The posttemporal is present in ictalurids and many other catfishes as a canal-bearing bone between the supracleithrum and the pterotic, a bone sometimes identified as the extrascapular. The extrascapular is missing in catfishes. Ictalurids have an additional dermal bone above the posttemporal, which is either an independently ossifying fragment of the posttemporal or a neoformation restricted to some members of this family. The single chondral bone of the pectoral girdle originates from a single center of ossification that represents the coracoid. The scapula is missing in catfishes. Dorsal-fin distal radial 2 is absent in catfishes and the foramen of dorsal-fin spine 2 is formed from modifications to the base of the fin-ray itself. Unlike loricarioid catfishes, the urohyal of ictalurids originates solely as an ossification of the sternohyoideus tendons. The anteriormost infraorbital element ossifies from a single center of ossification around the infraorbital sensory canal and represents the lacrimal. The antorbital is missing in catfishes. Finally, skeletal development of I. punctatus is compared to that available for other otophysans, including the cypriniforms Danio rerio and Enteromius holotaenia and the characiform Salminus brasiliensis.
Keywords
Catfish, Morphology, Ontogeny, Otophysi, Skeleton, Teleostei
Introduction
The order Siluriformes (catfishes) are a highly diverse (~3900 species,
Despite this remarkable morphological variation, Siluriformes are well characterized as a monophyletic group by several derived skeletal characters, including the secondary absence of several ossifications (e.g., subopercle, intercalar) and the extreme modification of others (e.g., anteriormost pectoral-fin ray, maxilla, metapterygoid) (
The highly modified skeleton of catfishes has been the subject of numerous detailed osteological investigations (e.g.,
In order to further our understanding of the catfish skeleton, as well as that of bony fishes more generally, I investigate skeletal development in the ictalurid catfishes Ictalurus punctatus and Noturus gyrinus. I compile a sequence of ossification for both species, documenting the progression of skeletal development (from the earliest stages of ossification through to later stages), and provide a high-quality photographic atlas illustrating select aspects of skeletal ontogeny for I. punctatus. Additionally, the homology of the five bones that have been proposed to represent compound elements in ictalurid catfishes (parieto-supraoccipital, posttemporo-supracleithrum, scapulocoracoid, urohyal, and lacrimal) are discussed in light of developmental information. Finally, a comparison is made between the ossification sequences produced herein for ictalurid catfishes and those available for other members of the Otophysi.
Methods
Eggs of Ictalurus punctatus were obtained from the Texas A&M Aquatic Research and Teaching Facility. Eggs were incubated until hatching, at which point embryos were moved to 5 gal aquaria where they were raised until sampling. Eggs were treated with Paraguard (Seachem Laboratories, Madison, GA) to prevent fungus. Embryos or larvae were sampled daily from 5 days pre-hatch up to 30 days post-hatch (dph) and every third day from 30 dph up to 60 dph. Sampled individuals were euthanized with an overdose of tricaine methanosulfate (MS222) and subsequently fixed in a solution of 10% buffered paraformaldehyde for 24 hr. After fixation, individuals were transferred to a 70% EtOH solution for permanent storage. Adult individuals (N = 8) of Noturus gyrinus were collected from the wild (Little Brazos River, Brazos Co., TX, USA) and brought back to the lab where they were sexed and divided (1–2 females per male) between 10 gallon aquaria (pH 7.5–8.0; temperature 26°C ± 1°C). Individuals were fed on a diet of decapsulated brine shrimp eggs, crushed blackworm pellets, and chopped blackworms and maintained for captive spawning. Upon spawning, eggs were collected, incubated, and sampled as described above for I. punctatus.
Gross and histological examination
A total of 100 individuals of Ictalurus punctatus (7.7 mm notochord length [NL] to 44.9 mm standard length [SL]) and 120 of Noturus gyrinus (5.4 mm SL to 26.4 mm SL) were cleared and double-stained (c&s) for bone and cartilage examination. Smaller specimens of I. punctatus (< 20 mm SL) were c&s using a modification of the acid-free clearing and staining method of
Once c&s, specimens were dissected and scored for the presence/absence of 328 (I. punctatus) and 286 (N. gyrinus) ossified skeletal elements under a ZEISS SteREO Discovery V20 stereomicroscope. For each individual specimen, bones were considered present at the first sign of alizarin red S staining and absent in the absence of alizarin red S staining. In the few cases in which it was not possible to confirm through stereomicroscopy whether a particular bone was stained with alizarin red S, specimens were examined at higher magnification using a Zeiss Primo Star compound microscope. The cartilage staining of
Neuromast staining
Variation in the number and position of canal neuromasts was studied in four members of the Ictaluridae (Ameiurus melas, Ictalurus punctatus, Noturus gyrinus, Pylodictis olivaris) and other catfishes representing the families Callichthyidae (Corydoras panda), Loricariidae (Ancistrus sp.), Mochokidae (Synodontis nigriventris), Pimelodidae (Pimelodus pictus), and Siluridae (Kryptopterus vitreolus). Specimens of aforementioned ictalurids were collected from the wild (Little Brazos River, Brazos Co., TX, USA) and members of other families were obtained via the ornamental aquarium fish trade. Canal neuromasts were stained in live individuals following the protocol of
Material examined
The following specimens, listed alphabetically by family, genus and species were examined during the course of this study. For each species, the collection numbers along with the total number of individuals from each lot examined and the size range of those specimens are listed. Individuals examined are whole mount c&s unless otherwise denoted. Institutional abbreviations follow
Amblycipitidae: Amblyceps cerinum, UMMZ 248835, 2 examined (ex.), 67.4–74.1 mm SL; Amblyceps mangois, UMMZ 244866, 2 ex., 36.5–37.8 mm SL; Liobagrus somjinensis, TCWC uncat., 1 ex., 42.8 mm SL. — Amphiliidae: Amphilius uranoscopus, CU 93740, 2 ex., 41.8–55.8 mm SL; CU 95213, 1 ex., 41.7 mm SL. — Anchariidae: Ancharius fuscus, AMNH 93702, 1 ex., 88.4 mm SL. — Ariidae: Ariopsis felis, TCWC 19690.02, 2 ex., 61.8–80.7 mm SL; Arius jordani, TCWC 19740.01, 2 ex., 50.0–51.1 mm SL; Bagre marinus, TCWC 547.07, 1 ex., 79.7 mm SL. — Aspredinidae: Bunocephalus sp., TCWC 19741.01, 2 ex., 33.8–43.4 mm SL; Pseudobunocephalus lundbergi, ANSP 168810, 5 ex., 20.1–22.7 mm SL. — Astroblepidae: Astroblepus sp., CU 78735, 2 ex., 32.1–54.4 mm SL; CU 78811, 2 ex., 27.8–30.0 mm SL. — Auchenipteridae: Tatia intermedia, TCWC 19752.01, 4 ex., 37.6–60.8 mm SL; Trachycorystes sp., FMNH 85945, 3 ex. , 55.7–65.8 mm SL. — Auchenoglanididae: Auchenoglanis occidentalis, CU 90478, 2 ex., 26.4–35.8 mm SL. — Austroglanididae: Austroglanis gilli, ANSP 177966, 1 ex., 71.8 mm SL — Bagridae: Pseudomystus siamensis, CAS 94782, 5 ex., 35.5–58.0 mm SL. — Callichthyidae: Corydoras panda, TCWC 19753.01, 6 ex., 8.7–18.9 mm SL; TCWC 20491.02, 1 stained for neuromast (neuro.), 32.0 mm SL. — Cetopsidae: Helogenes marmoratus, ANSP 175833, 1 ex., 50.7 mm SL; ANSP 177185, 4 ex., 30.9–36.7 mm SL. — Chacidae: Chaca chaca, UMMZ 208728, 1 ex., 156.0 mm SL. — Clariidae: Clarias batrachus, UMMZ 217578, 3 ex., 99.7–105.2 mm SL; Clarias gariepinus TCWC 15276.09, 2 ex., 62.5–71.3 mm SL. — Claroteidae: Chrysichthys mabusi, CU 91692, 2 ex., 58.0–80.7 mm SL. — Cranoglanididae: Cranoglanis bouderius, CAS-SU 69758, 1 ex., 97.0 mm SL. — Diplomystidae: Diplomystes chilensis, AMNH 55327, 1 ex., 64.4 mm SL; Diplomystes papillosus, CAS 81539, 1 ex., 118.0 mm SL. — Doradidae: Ossanocora punctata, TCWC 16723.16, 2 ex., 34.9–51.8 mm SL; Platydoras armatulus, TCWC 19754.01, 1 ex., , 45.1 mm SL. — Heptapteridae: Goeldiella eques, ANSP 177187, 2 ex., 99.2–104.5 mm SL. — Heteropneustidae: Heteropneustes fossilis, CAS 29627, 2 ex., 122.3–123.2 mm SL. — Horabagridae: Horabagrus brachysoma, TCWC 19755.01, 2 ex., 53.2–56.1 mm SL. — Ictaluridae: Ameirus melas TCWC 15355.08, 1 ex., 66.0 mm SL; TCWC 20490.01, 1 neuro., 45.0 mm SL; Ictalurus furcatus TCWC 19756.01, 4 ex., 64.9–70.8 mm SL; Ictalurus punctatus, TCWC 19757.01, 7 ex., 11.7–36.2 mm SL; TCWC 20490.02, 1 neuro., 60.0 mm SL; TCWC 20491.04, 100 ex., 7.7 mm NL–44.9 mm SL; TCWC 20491.05, 45 ex., 8.6 mm NL–21.2 mm SL; TCWC 20491.06, 1 skeletal preparation (skel), 426 mm SL; TCWC 20491.07, 1 skel, 436 mm SL; TCWC 20491.08, 1 skel, 441 mm SL; TCWC 20491.03, 1 pectoral fin sectioned, 30.1 mm SL; Noturus flavus UAIC 14314.07, 1 ex., 73.4 mm SL; Noturus gyrinus, TCWC 15438.13, 1 ex., 41.5 mm SL; TCWC 19758.01, 6 ex., 8.6–36.6 mm SL; TCWC 20490.03, 2 neuro., 25.0 mm SL–27.0 mm SL; TCWC 20491.10, 120 ex., 5.4 mm SL–26.4 mm SL; TCWC 20491.11, 38 ex., 5.8 mm SL–13.1 mm SL. Pylodictis olivaris TCWC 7834.10, 1 ex., 61.1 mm SL; TCWC 20490.04, 1 neuro., 56.0 mm SL. — Kryptoglanidae: Kryptoglanis shajii, BMNH uncat., 1 ex., 60.0 mm SL — Loricariidae: Ancistrus sp., TCWC 19759.01, 5 ex., 5.6–16.5 mm SL; TCWC 20491.01, 2 neuro., 11.0 mm SL–11.6 mm SL; Hemipsilichthys vestigipinnis, USNM 314657, 3 ex., 45.2–59.8 mm SL. — Malapteruridae: Malapterurus oguensis, CU 92271, 1 ex., 49.9 mm SL; CU 95140, 1 ex., 56.4 mm SL. — Mochokidae: Microsynodontis sp., TCWC 19760.01, 1 ex., 26.2 mm SL; Synodontis sp., TCWC 20491.13, 2 neuro., 24.0 mm SL–25.0 mm SL. — Nematogenyidae: Nematogenys inermis, USNM 84343, 1 ex., 25.8 mm SL. — Pangasiidae: Pangasius macronema, CAS 29360, 3 ex., 50.5–66.5 mm SL; UMMZ 214029, 2 ex., 103.9–104.7 mm SL. — Pimelodidae: Pimelodus ornatus, LACM 41735.022; LACM 41740.015; Pimelodus pictus, TCWC 19761.01, 2 ex., 33.1–37.6 mm SL; TCWC 20491.12, 1 neuro., 42.0 mm SL. — Plotosidae: Plotosus lineatus, FMNH 110269, 5 ex., 21.0–66.7 mm SL. — Pseudopimelodidae: Microglanis poecilus, AMNH 54973, 2 ex., 23.1–23.9 mm SL. — Ritidae: Rita rita, CAS-SU 34866, 1 ex., 85.0 mm SL. — Schilbeidae: Parailia congica, AMNH 246178, 2 ex., 60.5–60.8; Parailia pellucida, USNM 229794, 3 ex., 29.9–32.3 mm SL; Schilbe intermedius TCWC 15286.18, 3 ex., 57.6–72.7 mm SL. — Scoloplacidae: Scoloplax empousa, FMNH 108610, 5 ex., 12.8–19.1 mm SL. — Siluridae: Kryptopterus sp., TCWC 20491.09, 1 neuro., 43.0 mm SL; Silurus asotus, ANSP 185139, 3 ex., 51.2–67.5 mm SL; Silurus glanis, BMNH 2005.7.5.944–1034, 4 ex., 17.2–85.0 mm SL; Wallago attu, CAS 92824, 2 ex., 69.2–71.0. — Sisoridae: Glyptothorax sinensis, UMMZ 246438, 1 ex., 60.7 mm SL; Parachiloglanis hodgarti, CAS 50170; KU 29549; KU 40556; Pseudolaguvia kapuri, CAS 50294, 4 ex., 23.4–26.6 mm SL. — Trichomycteridae: Henonemus sp., TCWC 13989.19, 1 ex., 69.7 mm SL; Trichomycterus hasemani, ANSP 175851, 3 ex., 13.2–13.9 mm SL.
Results
Overview of skeletal development
Approximately 328 and 286 individual elements (not including individual fin rays, gill rakers, or parapophyses) are present in the skeleton of Ictalurus punctatus and Noturus gyrinus, respectively. The total number of skeletal elements considered herein was condensed to 143 elements in I. punctatus and 137 elements in N. gyrinus by treating multiple serially repetitive elements as a single element. This included branchiostegal rays, vertebral elements posterior to vertebra 5 (excluding parapophyses and ribs) and anterior to preural vertebra 3, dorsal- and anal-fin proximal and distal radials (excluding dorsal-fin proximal radials 1–3), and post-Weberian ribs. All elements of the skeleton are ossified by 22.4 mm SL in I. punctatus (Fig.
Ossification sequence of 143 skeletal elements of Ictalurus punctatus. Black bars along horizontal axis represent the length at which a particular ossification is present in all individuals (fixed). Error bars associated with black bars indicate the length at which a particular ossification is present in some but not all individuals. Vertical axis represents length in mm NL/SL.
Ossification sequence of 137 skeletal elements of Noturus gyrinus. Black bars along horizontal axis represent the length at which a particular ossification is present in all individuals (fixed). Error bars associated with black bars indicate the length at which a particular ossification is present in some but not all individuals. Vertical axis represents length in mm SL.
Skeletal development of Ictalurus punctatus
In the following sections, I provide a detailed overview of skeletal development in Ictalurus punctatus. A previous description of development for the post-Weberian axial skeleton in I. punctatus was provided by
Ossification sequence of 143 skeletal elements of Ictalurus punctatus separated by skeletal region. Black bars along horizontal axis represent the length at which a particular ossification is present in all individuals (fixed). Error bars associated with black bars indicate the length at which a particular ossification is present in some but not all individuals. Vertical axis represents length in mm NL/SL.
Ossification sequence of 137 skeletal elements of Noturus gyrinus separated by skeletal region. Black bars along horizontal axis represent the length at which a particular ossification is present in all individuals (fixed). Error bars associated with black bars indicate the length at which a particular ossification is present in some but not all individuals. Vertical axis represents length in mm SL.
Neurocranium ethmoid region
The most common sequence of ossification: nasal (11.4 mm SL) – mesethmoid (12.2 mm SL) – lateral ethmoid (12.8 mm SL) – vomer (14.5 mm SL) (Figs
Ontogeny of the neurocranium of Ictalurus punctatus in dorsal view. A 10.9 mm SL. B 12.2 mm SL. C 13.3 mm SL. D 18.0 mm SL. E 21.2 mm SL. F 44.9 mm SL. Abbreviations: *, Accessory posttemporal; Asph, Autosphenotic; Dpto, Dermopterotic; EpBar, Epiphysial bar; Epoc, Epioccipital; EthPla, Ethmoid plate; Fr, Frontal; LamOrbN, Lamina orbitonasalis; LE, Lateral ethmoid; ME, Mesethmoid; Na, Nasal; OtCap, Otic capsule; Pt, Posttemporal; Pto, Pterotic; Soc, Supraoccipital; SphCom, Sphenoseptalis commissure; TMA, Taenia marginalis anterior; TMP, Taenia marginalis posterior.
Nasal. The nasal is a dermal ossification that first appears in some individuals of 10.9 mm SL (Figs
Ontogeny of the neurocranium of Ictalurus punctatus in left lateral view. A 10.9 mm SL. B 12.2 mm SL. C 13.3 mm SL. D 18.0 mm SL. E 21.2 mm SL. F 44.9 mm SL. Abbreviations: *, Accessory posttemporal; Asph, Autosphenotic; Boc, Basioccipital; Dpto, Dermopterotic; EpBar, Epiphysial bar; Epoc, Epioccipital; EthPla, Ethmoid plate; Exoc, Exoccipital; Fr, Frontal; LamOrbN, Lamina orbitonasalis; LE, Lateral ethmoid; ME, Mesethmoid; Na, Nasal; OpF, Foramen for the passage of the optic nerve (II); Orsph, Orbitosphenoid; OtCap, Otic capsule; OTF, Common foramen for the passage of the optic (II), trigeminal (V), and Facial (VII) nerves; PrF, Preoptic fontanelle; Pro, Prootic; Psph, Parasphenoid; Pt, Posttemporal; Pto, Pterotic; Ptsph, Pterosphenoid; Soc, Supraoccipital; SphCom, Sphenoseptalis commissure; TMA, Taenia marginalis anterior; TMP, Taenia marginalis posterior; TrCran, Trabecula cranii; TrFaF, Common foramen for the passage of the trigeminal (V) and facial nerves (VII); TS, Tectum synoticum; Vo, Vomer.
Mesethmoid. The mesethmoid first appears at 11.9 mm SL as a paired perichondral ossification located along the anteriodorsal edge of the ethmoid cornua of the ethmoid plate. By 13.2 mm SL (Figs
Ontogeny of the neurocranium of Ictalurus punctatus in ventral view. A 10.9 mm SL. B 12.2 mm SL. C 13.3 mm SL. D 18.0 mm SL. E 21.2 mm SL. F 44.9 mm SL. Asph, Autosphenotic; Ast, Asteriscus; Boc, Basioccipital; Epoc, Epioccipital; EthPla, Ethmoid plate; Exoc, Exoccipital; Fr, Frontal; LamOrbN, Lamina orbitonasalis; Lap, Lapillus; LE, Lateral ethmoid; ME, Mesethmoid; Na, Nasal; Orsph, Orbitosphenoid; OtCap, Otic capsule; Pro, Prootic; Psph, Parasphenoid; Pto, Pterotic; Ptsph, Pterosphenoid; Sag, Sagitta; Soc, TMA, Taenia marginalis anterior; TMP, Taenia marginalis posterior; TrCran, Trabecula cranii; Vo, Vomer.
Ictalurus punctatus, Neurocranium of specimen TCWC 20491.06, 426 mm SL, in dorsal A, lateral B and ventral view C. Abbreviations: *, Accessory posttemporal; Asph, Autosphenotic; Boc, Basioccipital; Epoc, Epioccipital; Exoc, Exoccipital; Fr, Frontal; LE, Lateral ethmoid; ME, Mesethmoid; Na, Nasal; OpF, Foramen for the passage of the optic nerve (II); Orsph, Orbitosphenoid; PrF, Preoptic fontanelle; Pro, Prootic; Psph, Parasphenoid; Pt, Posttemporal; Pto, Pterotic; Ptsph, Pterosphenoid; Soc, Supraoccipital; TrFaF, Common foramen for the passage of the trigeminal (V) and facial nerves (VII); Vo, Vomer.
Lateral Ethmoid. The paired lateral ethmoid first appears (12.4 mm SL) as a perichondral ossification of the lamina orbitonasalis at its mid-length near the orbitonasal foramen. At 13.4 mm SL the ossification has expanded on both the anterior and posterior surface of the lamina orbitonasalis, which is pierced by the orbitonasal foramen, which is now completely surrounded by bone. The lateral ethmoid continues to spread towards the taenia marginalis anterior and sphenoseptalis commissure dorsally and the ethmoid plate ventrally, and extends around the lateral edge of the lamina orbitonasalis by 15.9 mm SL. By 18.0 mm SL (Figs
Vomer. The dermal vomer first appears in some individuals as small as 13.2 mm SL (Figs
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: nasal (7.2 mm SL) – mesethmoid (8.3 mm SL) – lateral ethmoid (8.7 mm SL) – vomer (10.5 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the ethmoid region of the neurocranium, which is similar in both species and show no major differences in adult morphology.
Neurocranium orbital region
The most common sequence of ossification: parasphenoid (10.0 mm SL) – frontal (11.3 mm SL) – pterosphenoid and orbitosphenoid (13.4 mm SL) (Figs
Parasphenoid. The parasphenoid first appears in individuals of 10.0 mm SL as a thin plate of bone of bone running medial to the trabeculae cranii and ventral to a portion of the hypophyseal foramen. By 11.3 mm SL, the parasphenoid has expanded anteriorly and posteriorly, now covering the entirety of the hypophyseal foramen, with both ends tapering in width giving the bone a rhomboid appearance. Posteriorly it extends across the ventral surface of the otic capsule where it stops ventral to the anterior tip of the notochord and anteriorly it reaches the point of the lamina orbitonasalis. Two small ascending processes, represented by thin laminae of bone, have formed along the lateral margin near the widest point of the bone. At 13.3 mm SL (Figs
Frontal. The dermal frontal first appears (10.9 mm SL; Figs
Pterosphenoid. The pterosphenoid first appears at 12.7 mm SL as a paired perichondral ossification on the dorsal margin of the common foramen for the passage of the optic (II), trigeminal (V) and facial (VII) nerves, and becomes fixed in development at 13.4 mm SL. By 14.2 mm SL, the perichondral ossification has expanded in size to become semicircular in shape. Membrane bone processes extend ventrally from the perichondral ossification leaving two openings, which (by 15.9 mm SL in most specimens) become surrounded by bone forming the foramina for the passage of the ophthalmic branch of the trigeminal and facial nerves. The bone continues to expand in all directions and by 21.2 mm SL (Figs
Orbitosphenoid: The paired orbitosphenoid originates at 12.8 mm SL as a small perichondral ossification along the anterior margin of the common foramen for the passage of the Optic (II), Trigeminal (V) and Facial (VII) nerves, and becomes fixed in development at 13.4 mm SL. The perichondral ossification expands across the cartilage anteriorly and becomes crescentic in appearance by 14.2 mm SL. As the orbitosphenoid grows, it eventually meets its antimere ventrally and the two elements fuse into a single ossification (16.2 mm SL). At 18.0 mm SL (Fig.
Comparison with Noturus gyrinus: The most common sequence of ossification for this region in Noturus gyrinus is as follows: frontal (6.6 mm SL) – parasphenoid (7.0 mm SL) – pterosphenoid and orbitosphenoid (9.5 mm SL).
The only difference in the sequence of ossification identified between Noturus gyrinus and Ictalurus punctatus in the orbital region of the neurocranium is that the frontal appears before the parasphenoid while in I. punctatus it appears after the parasphenoid. The orbital region of N. gyrinus and I. punctatus are otherwise similar, and no major differences in adult morphology are observed in this region.
Neurocranium otic region
The most common sequence of ossification: prootic (11.7 mm SL) – pterotic (12.2 mm SL) – autosphenotic (13.4 mm SL) (Figs
Prootic. The paired chondral prootic is one of the largest bones in the neurocranium. It first appears at 11.5 mm SL as a perichondral ossification on the ventrolateral surface of the otic capsule ventral to the utricular capsule, and is found in all individuals of 11.7 mm SL or larger. The prootic has started to ossify endochondrally and reaches the posterior edge of the common foramen for the passage of the optic (II), trigeminal (V) and facial (VII) nerves anteriorly (13.3 mm SL; Fig.
Pterotic. The compound pterotic is composed of both chondral (autopterotic) and dermal (dermopterotic) bones. The dermopterotic is the first to appear (11.9 mm SL) on the posterolateral surface of the otic capsule as two thin, trough shaped ossifications of the pterotic portion of the otic sensory canal. By 13.3 mm SL (Figs
Autosphenotic. The autosphenotic first appears at 13.1 mm SL as a perichondral ossification at the junction of the taenia marginalis and the otic capsule. By 14.2 mm SL, the autosphenotic has started to endochondrally ossify and by 15.4 mm SL it covers the anteroventral border of the anterior vertical semicircular canal and has expanded dorsally to the anterodorsal edge of the otic capsule where it meets the frontal. At this stage, a trough shaped ossification of membrane bone arises from the autosphenotic along the sphenotic portion of the otic sensory canal. The roof of the sensory canal has started to form in bone by 17.8 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: prootic (8.4 mm SL) – pterotic (7.8 mm SL) – autosphenotic (8.7 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the otic region of the neurocranium, which is similar in both species and show no major differences in adult morphology.
Neurocranium occipital region
The most common sequence of ossification: basioccipital (10.0 mm SL) – exoccipital (11.4 mm SL) – posttemporal (12.8 mm SL) – supraoccipital (12.9 mm SL) – epioccipital (14.8 mm SL) – accessory posttemporal (15.1 mm SL) (Figs
Basioccipital. The basioccipital originates as a perichordal ossification around the anterior tip of the notochord at the base of the cranium (10.0 mm NL). By 12.0 mm SL (Figs
Exoccipital. The paired exoccipital first appears at 10.9 mm SL (Figs
Posttemporal. The posttemporal can first be observed in 12.4 mm SL individuals as a small weakly ossified trough of bone around the lateral line sensory canal anterior to the supracleithrum and in line with the posterior margin of the chondrocranium. By 16.2 mm SL, the roof of the sensory canal has begun to close with bone and by 21.2 mm SL (Figs
Supraoccipital. Whether this element is of compound origin (parietal+supraoccipital) or not has been a contentious subject in the past. Herein I refer to the element as the supraoccipital and further discuss the homology of this bone in the discussion. The supraoccipital originates as a pair of perichondral ossifications in the tectum synoticum on either side of the posterior cranial fontanelle (12.5 mm SL first appearance, 12.9 mm SL fixed length). Soon after (13.3 mm SL; Figs
Epioccipital. The paired chondral epioccipital first appears at 14.6 mm SL in some individuals as a small circular perichondral ossification of the posterior otic capsule just ventral to the tip of the anterior arm of the supracleithrum. It has started to endochondrally ossify by 15.6 mm SL and it continues to increase in size and extends ventrally, becoming more ovoid and covering the posteroventral portion of the posterior vertical semicircular canal of the inner ear (21.2 mm SL; Figs
Accessory posttemporal. The homology of this element located on the dorsolateral surface of the cranium in some ictalurids has been contentious in the past (see
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: basioccipital (5.9 mm SL) – exoccipital (6.4 mm SL) – posttemporal (8.0 mm SL) – supraoccipital (8.6 mm SL) – epioccipital (10.4 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the occipital region of the neurocranium. The occipital region of N. gyrinus differs slightly from that of I. punctatus in that the accessory posttemporal is absent and the posttemporal consists of only a canal ossification with no laminar portion.
Jaws
The most common sequence of ossification: maxilla (8.6 mm NL) – dentary (9.3 mm NL) – premaxilla (10.4 mm SL) – retroarticular (12.2 mm SL) – anguloarticular (12.7 mm SL) – coronomeckelian (15 mm SL) (Fig.
Ontogeny of the hyopalatine arch, jaws and opercular series of Ictalurus punctatus. A 11.2 mm SL. B 12.2 mm SL. C 13.3 mm SL. D 18.0 mm SL. E 21.5 mm SL. F Suprapreopercle not shown, 44.9 mm SL. Abbreviations: Ana, Anguloarticular; Ana-Ra, Anguloarticular+retroarticular; Apa, Autopalatine; Cm, Coronomeckelian; De, Dentary; Enpt, Endopterygoid; Hy, Hyomandibular; Iop, Interopercle; MC, Meckel’s cartilage; Mpt, Metapterygoid; Mx, Maxilla; MxBC, Mandibular barbel cartilage; Op, Opercle; PA, Pars autopalatina; PHy, Pars hyomandibularis; PMpt, Pars metapterygoidea; Pmx, Premaxilla; Pop, Preopercle; PQ, Pars quadrata; Q, Quadrate; Ra, Retroarticular; Sop, Subopercle; Spop, Subpreopercular bone.
Maxilla. The paired maxilla is one of the first three elements to ossify in the skeleton of Ictalurus punctatus (8.6 mm NL). It starts off as a slightly curved lamina located anteriorly to the pars autopalatina that extends laterally to cover the anteroproximal tip of the maxillary barbel cartilage. By 11.3 mm SL (Fig.
Dentary. The dentary originates early in development, appearing in specimens as small as 8.7 mm NL as a dermal lamina of bone lateral to Meckel’s cartilage. The first few teeth can be seen in specimens of 10.4 mm SL at which point the dentary extends across the length of Meckel’s cartilage up to the coronoid process. Shortly after this (11.2 mm SL; Fig.
Premaxilla. The paired premaxilla originates as small splint of bone located ventral to the tips of the ethmoid cornua (10.1–10.4 mm SL) with the first teeth appearing shortly after this (11.5 mm SL). By 12.4 mm SL (Fig.
Ictalurus punctatus, specimen TCWC 20491.08, 441 mm SL (A), (B), (C), (E), and TCWC 20491.07, 436 mm SL (D). A Hyopalatine arch, jaws and opercular series in lateral view. B Lower jaw in medial view. Area outline by white box indicates location of (C). C Coronomeckelian on medial surface of the lower jaw. D Hyoid bar in lateral view. E Ventral gill arches in dorsal view. F Dorsal gill arches in dorsal view. G Dorsal gill arches in ventral view. Abbreviations: ACh, Anterior ceratohyal; Ana-Ra, Anguloarticular+retroarticular; Apa, Autopalatine; Bb, Basibranchial; Br, Branchiostegal ray; Cb, Ceratobranchial; Cm, Coronomeckelian; De, Dentary; DHh, Dorsal hypohyal; Eb, Epibranchial; Enpt, Endopterygoid; Hb, Hypobranchial; Hy, Hyomandibular; Ih, Interhyal;Iop, Interopercle; Mpt, Metapterygoid; Mx,Maxilla; Op, Opercle; Pb, Pharyngobranchial; PCh, Posterior ceratohyal; Pmx, Premaxilla; Pop, Preopercle; Q, Quadrate; Sop, Subopercle; Supop, Suprapreopercle; TPCb, Toothplate of ceratobranchial; TPPb, Toothplate of pharyngobranchial;Uh, Urohyal; VHh, Ventral hypohyal.
Retroarticular. The retroarticular is first observed in specimens of 11.6 mm SL as a perichondral ossification at the point of attachment of the interoperculo-retroarticular ligament on the posteroventral-most tip of Meckel’s cartilage. The retroarticular becomes more heavily ossified around the posterior tip of Meckel’s cartilage and around 13.0 mm SL (Fig.
Anguloarticular. The anguloarticular is a compound element composed of the dermal angular and the endoskeletal articular, although the two bones were not observed as separate ossifications. The element first appears as an ossification on the dorsal edge of Meckel’s cartilage posterior to the coronoid process (11.9 mm SL). The anguloarticular ossifies in a dorsoventral direction becoming saddle-shaped in appearance at 12.5 mm SL. At 13.0 mm SL the bone completely encompasses the posterior end of Meckel’s cartilage (Fig.
Coronomeckelian. The coronomeckelian is a small dermal bone that is first observed as a tiny ossification located medially to the coronal process of Meckel’s cartilage at the tendinous insertion of the A3 adductor mandibulae (15.0 mm SL). It becomes triangular in shape shortly after ossifying (16.2 mm SL) and maintains this shape and position during its early ontogeny, only increasing in size (44.9 mm SL; Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: maxilla (5.4 mm NL)– dentary (5.9 mm NL) – premaxilla (6.6 mm SL) – retroarticular (7.0 mm SL) – anguloarticular (7.7 mm SL) – coronomeckelian (10.1 mm SL).
No differences in the sequence of ossification between Noturus gyrinus and Ictalurus punctatus were identified in the jaws. The jaws of N. gyrinus and I. punctatus are similar, and no major differences in adult morphology are observed in this region.
Hyopalatine arch
The most common sequence of ossification: quadrate (11.5 mm SL) – hyomandibular (12.0 mm SL) – metapterygoid (12.8 mm SL) – endopterygoid and autopalatine (12.9 mm SL) (Fig.
Quadrate. the quadrate is an endoskeletal bone that first appears in some individuals of 11.2 mm SL (Fig.
Hyomandibular. The hyomandibular originates as a perichondral ossification around the pars hyomandibularis near the foramen for the passage of the hyomandibular branch of the facial nerve (VII) and may appear as early as 11.5 mm SL. By 13.2 mm SL (Fig.
Metapterygoid. The metapterygoid starts off as a perichondral ossification around the middle of the anterior process of the pars metapterygoidea (11.9 mm SL), which in catfishes has rotated and shifted anteriorly towards the pars autopalatina. By 13.3 mm SL (Fig.
Endopterygoid. The endopterygoid first appears as a thin splint of bone in the ligamentous connection between the metapterygoid, autopalatine and neurocranium in individuals as small as 11.9 mm SL. The bone expands into a thin lamina of bone (14.1 mm SL) and by 15.6 mm SL it has become large enough to replace the portion of the ligament in which it has formed resulting in three ligamentous connections to the metapterygoid posteriorly, the autopalatine laterally and the vomer anteriorly. An additional, second ligament connecting the endopterygoid to the vomer was previously reported (
Autopalatine. The autopalatine starts as a perichondral ossification (12.5 mm SL) around the middle of the cylindrical pars autopalatina, which originates as an independent cartilage separate from the rest of the palatoquadrate. By 13.9 mm SL it has expanded into a cylindrical ossification around the middle third of the pars autopalatina except for a dorsomedial facet for the articulation with the lateral ethmoid. Anteriorly, the cartilaginous head of the autopalatine possesses a ventrolateral groove for the articulation of the maxilla. The pars autopalatina has increased in size with the posterior tip extending almost to the metapterygoid by 18.0 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: quadrate and hyomandibular (7.2 mm SL) – metapterygoid (8.3 mm SL) – autopalatine (8.6 mm SL) – endopterygoid (8.7 mm SL).
The sequence of ossification in the hyopalatine arch differs between Noturus gyrinus and Ictalurus punctatus in that the quadrate appears before the hyomandibular in I. punctatus while in N. gyrinus they are fixed in development at the same size. However, the quadrate does appear before the hyomandibular in some individuals of N. gyrinus. Additionally, the autopalatine appears before the endopterygoid while in I. punctatus, despite being fixed in development at the same size, the endopterygoid is present before the autopalatine in some individuals. The hyopalatine arch of N. gyrinus and I. punctatus are similar, and no major differences in adult morphology are observed in this region.
Opercular series
The most common sequence of ossification: opercle (8.6 mm NL) – interopercle (10.4 mm SL) – preopercle (11.5 mm SL) – suprapreopercle (22.4 mm SL) (Fig.
Opercle. The dermal opercle is one of the first skeletal elements to appear in Ictalurus punctatus (8.6 mm NL) and was first observed as a thin dermal ossification extending posteroventrally from the posterior condyle of the pars hyomandibularis of the hyosymplectic cartilage. At 9.6 mm NL, the posterior end of the ossification begins to widen and by 10.8 mm SL the opercle has become fan-shaped with a concave anterior and dorsal edge, the latter of which is more heavily ossified. As the opercle continues to expand, its anteroventral margin becomes more rounded as it gets closer to the posterior margin of the interopercle while its posterior dorsal tip ends in a sharp point (13.0 mm SL; Fig.
Interopercle. The dermal interopercle first appears at 10.4 mm SL as a small splint of bone lateral to the connection between the ceratohyal and interhyal cartilages. By 13.3 mm SL (Fig.
Preopercle. The preopercle forms as a splint of bone (10.9 mm SL) along the posterior margin of the hyosympletic and pars quadrata cartilages at the point of contact with the interhyal cartilage. At 12.2 mm SL (Fig.
Suprapreopercle. The suprapreopercle is the last element to appear in the entire skeleton of Ictalurus punctatus (22.4 mm SL) and is first observed as a small ossification located between the preopercle and the pterotic. At 44.9 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: opercle (5.4 mm NL) – interopercle (6.4 mm SL) – preopercle (7.7 mm SL) – suprapreopercle (11.6 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the opercular series, which is similar in both species and show no major differences in adult morphology
Infraorbitals
The most common sequence of ossification: lacrimal (10.4 mm SL) – infraorbital 2 (12.2 mm SL) – infraorbital 3 (12.8 mm SL) – infraorbital 4 and 6 (13.2 mm SL) – infraorbital 5 (13.9 mm SL) (Fig.
Lacrimal. The lacrimal first appears at 10.4 mm SL as a small dermal bone located dorsal to the articulation between the maxilla and pars autopalatina and anterior to the lamina orbitonasalis (Fig.
Infraorbital 2. Infraorbital 2 first appears as small trough-shaped bone just posterior to the lacrimal at 11.9 mm SL. A single foramen for innervation of neuromasts in the infraorbital sensory canal is present in the center of the ossification. At 13.2 mm SL (Fig.
Infraorbital 3. Infraorbital 3 first appears (11.9 mm SL, Fig.
Infraorbital 4. Infraorbital 4 first appears (in some individuals as small as 12.4 mm SL) as a small lamina of bone around a foramen for the innervation of neuromasts ventral to the anterior quarter of the eye. It becomes trough shaped shortly after this (13.1 mm SL; Fig.
Infraorbital 6. Infraorbital 6 is the largest infraorbital in the series. It appears directly ventral to the junction of the supraorbital, infraorbital and otic sensory canals near the vertical midline of the eye and at approximately the same time as infraorbital 4 (12.4 mm SL). The canal becomes enclosed in bone by 14.1 mm SL and the bone continues to increase in length. By 44.9 mm SL (Fig.
Infraorbital 5. Infraorbital 5 forms around a foramen for the innervation of neuromasts in individuals as small as 13.2 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: lacrimal (7.0 mm SL) – infraorbital 2 (7.7 mm SL) – infraorbital 3 (7.9 mm SL) – infraorbital 4 (8.3 mm SL) – infraorbital 5 (8.6 mm SL) – infraorbital 6 and 7 (12 mm SL).
The only difference observed in the sequence of ossification between Noturus gyrinus and Ictalurus punctatus in the infraorbital series is that infraorbital 5 appears before infraorbital 6 in N. gyrinus while it appears after infraorbital 6 in I. punctatus. The infraorbitals of N. gyrinus and I. punctatus are generally similar except for N. gyrinus possessing an additional element, infraorbital 7. The only difference noted is the shape of the lacrimal in N. gyrinus in which the anterior process in more elongate and the posterior process is a narrow splint that does not reach the lamina orbitonasalis.
Hyoid bar
The most common sequence of ossification: branchiostegal ray 8 (9.6 mm NL) – branchiostegal ray 7 (10.0 mm SL) – branchiostegal ray 6 (10.8 mm SL) – anterior ceratohyal (11.3 mm SL) – branchiostegal ray 5 (11.4 mm SL) – branchiostegal ray 4, urohyal and ventral hypohyal (11.9 mm SL) – branchiostegal ray 3 (12.3 mm SL) – branchiostegal ray 2 (12.8 mm SL) – interhyal and posterior ceratohyal (13.2 mm SL) – branchiostegal ray 1 (13.6 mm SL) – dorsal hypohyal (15.0 mm SL) (Fig.
Ontogeny of the hyoid bar of Ictalurus punctatus. A 10 mm NL. B 12.5 mm SL. C 13.3 mm SL. D 15.0 mm SL. E 44.9 mm SL. Abbreviations: ACh, Anterior ceratohyal; Br, Branchiostegal ray; DHh, Dorsal hypohyal; Ih, Interhyal; IhC, Interhyal cartilage; PCh, Posterior ceratohyal; Uh, Urohyal; VHh, Ventral hypohyal.
Branchiostegal Rays. The branchiostegal rays appear as thin dermal ossifications extending posteroventrally from the hyoid bar. The first branchiostegal ray to develop is the posteriormost, branchiostegal ray 8 (9.2 mm NL), along the posteroventral margin of the deepest portion of the ceratohyal cartilage. The next three branchiostegal rays to appear are branchiostegal rays 7 (10.0 mm SL; Fig.
Anterior Ceratohyal. The anterior ceratohyal develops (10.8 mm SL) as a perichondral ossification around the middle of the slender anterior portion of the ceratohyal cartilage. The cylindrical ossification extends anteriorly and posteriorly until it covers the middle third of the ceratohyal cartilage (12.5 mm SL; Fig.
Urohyal. The urohyal originates as a pair of ossifications in the posterior portion of the sternohyoideus tendons that insert on the medial surface of the anterior most point of the ceratohyal cartilage (11.2 mm SL). The two ossifications expand in a fan-like direction posteriorly and fuse by 12.2 mm SL, forming a sheet of bone ventrally. By 13.2 mm SL (Fig.
Ventral Hypohyal. The paired, chondral, ventral hypohyal develops (11.2 mm SL) ventrally on the medioventral process of the anterior head of the ceratohyal cartilage that supports the insertion of the sternohyoideus tendons. At 12.5 mm SL (Fig.
Interhyal. The small interhyal cartilage forms a connection between, and is continuous with, the ceratohyal and the pars quadrata-hyomandibularis cartilages. The interhyal first appears as perichondral ossification around the interhyal cartilage in some individuals as small as 12.5 mm SL, shortly after the cartilaginous connections with the ceratohyal and pars quadrata-hyomandibularis cartilage begins to regress. By 14.0 mm SL, the perichondral ossification covers most of the interhyal cartilage which is now an independent cartilage with connective tissue replacing its previously cartilaginous dorsal and ventral connections. It continues to become more heavily ossified (21.2 mm SL) and by 44.9 mm SL (Fig.
Posterior Ceratohyal. The posterior ceratohyal develops in individuals as small as 12.5 mm SL as an ossification on the lateral surface of the ceratohyal cartilage just anterior to its ligamentous connection with the interopercle. Shortly after (13.1 mm SL; Fig.
Dorsal Hypohyal. the dorsal hypohyal originates as a perichondral ossification medially on the posterodorsal process of the anterior head of the ceratohyal cartilage (14.5 mm SL). The bone is saddle shaped (15.9 mm SL) and slowly expands to incorporate the entire medial corner of the posterodorsal process (21.5 mm SL). By 44.9 mm SL, the dorsal hypohyal has changed little in shape but has expanded ventrally towards the ventral hypohyal, from which it remains separated by cartilage. In adult individuals (436 mm SL), the dorsal hypohyal covers the entire posterodorsal process which now ends in a distinct point giving the bone a conical appearance.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: branchiostegal ray 9 (6.3 mm NL) – branchiostegal ray 8 (6.4 mm NL) – branchiostegal ray 7 (6.7 mm SL) – anterior ceratohyal (7.0 mm SL) – branchiostegal ray 5 and 6 (7.3 mm SL) – urohyal (7.3 mm SL) – ventral hypohyal (7.6 mm SL) – posterior ceratohyal (7.7 mm SL) – branchiostegal ray 3 and 4 (7.9 mm SL) – branchiostegal ray 2 and 1 (8.6 mm SL) – dorsal hypohyal (10.8 mm SL) – interhyal (11.1 mm SL).
The sequence of ossification in the hyoid bar differs between Noturus gyrinus and Ictalurus punctatus in that the interhyal is the last element to appear in N. gyrinus while in I. punctatus it appears before the dorsal hypohyal at the same time as the posterior ceratohyal. The hyoid bar of N. gyrinus and I. punctatus differ in the total number of branchiostegal rays (9 in N. gyrinus vs. 8 in I. punctatus). Although development of the branchiostegal rays also occurs in a posterior to anterior direction, the precise sequence of appearance was not as well resolved in N. gyrinus.
Branchial skeleton
The most common sequence of ossification: pharyngobranchial 4 toothplate (9.9 mm NL) – ceratobranchial 5 toothplate (10.9 mm SL) – ceratobranchial 1, 2, 3, 4, 5 and epibranchial 4 (12.0 mm SL) – epibranchial 1, 2 and 3 (12.1 mm SL) – gill rakers (13.3 mm SL) – pharyngobranchial 3 (14.5 mm SL) – pharyngobranchial 4 (15.0 mm SL) – basibranchial 2 and 3 (15.9 mm SL) – hypobranchial 1 (18.0 mm SL) – hypobranchial 2 (21.2 mm SL) (Fig.
Ontogeny of the branchial skeleton of Ictalurus punctatus. Ventral and dorsal gill arches shown in left and right column respectively. A 10.9 mm SL. B 12.9 mm SL. C 15.0 mm SL. D 20.6 mm SL. Abbreviations: AC, Anterior copula; Bb, Basibranchial; BbC, Basibranchial cartilage; Cb, Ceratobranchial; Cb, Ceratobranchial cartilage; Eb, Epibranchial; EbC, Epibranchial cartilage; Hb, Hypobranchial; HbC, Hypobranchial cartilage; Pb, Pharyngobranchial; PbC, Pharyngobranchial cartilage; PC, Posterior copula; TPCb, Toothplate of ceratobranchial; TPPb, Toothplate of pharyngobranchial.
Ceratobranchials. The ceratobranchials start as perichondral ossifications around the middle of their respective cartilages. The first to ossify is ceratobranchial 4 (11.4 mm SL) with the remaining ceratobranchials ossifying shortly after (11.8 mm SL). The perichondral ossification expands towards the tips of the cartilages and the teeth associated with the lower pharyngeal jaws are ankylosed to ceratobranchial 5 toothplate which has already fused with ceratobranchial 5 (12.9 mm SL; Fig.
Epibranchials. Epibranchials 1–4 ossify perichondrally around the midline of the epibranchial cartilages and, like in the ceratobranchials, ossification proceeds to spread across the entirety of the cartilages, excluding the tips which remain cartilaginous. The epibranchials ossify rapidly with all of them first appearing in individuals as small as 11.9 mm SL with epibranchial 4 being the first to become fixed in development at 12.0 mm SL. Additionally a posterior to anterior sequence of epibranchial ossification is suggested by a single specimen (11.9 mm SL) in which only epibranchials 3 and 4 are present. By 15.0 mm SL (Fig.
Gill Rakers. The gill rakers first appear on ceratobranchial 1 in individuals as small as 12.7 mm SL and by 13.3 mm SL, at least one gill raker is associated with each of the five ceratobranchials. By 15.9 mm SL, 5–6 gill rakers are present on each ceratobranchial and a single gill raker is associated with epibranchials 1 and 2. At 20.6 mm SL (Fig.
Pharyngobranchials. Only two pharyngobranchial cartilages, those of arches 3 and 4, are present in Ictalurus punctatus. Teeth associated with pharyngobranchial 4 toothplate can be seen in individuals as small as 9.9 mm SL ventral to the pharyngobranchial 4 cartilage (Fig.
Basibranchials. Only two basibranchials, basibranchial 2 and 3, are present in Ictalurus punctatus. These form as perichondral bands of bone around the middle and posterior end of the anterior basibranchial copula. Both ossifications appear at approximately the same time with basibranchial 2 appearing slightly earlier (basibranchial 2, 14.6 mm SL; basibranchial 3, 14.8 mm SL). The basibranchials become more elongate (20.6 mm SL; Fig.
Hypobranchials. The hypobranchials are some of the last bones to ossify in Ictalurus punctatus. Hypobranchial 1 first appears at 15.1 mm SL and hypobranchial 2 at 17.7 mm SL. Both start as perichondral ossifications at the anterolateral tips of hyobranchial cartilages 1 and 2. By 20.6 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: pharyngobranchial 4 toothplate (6.6 mm NL) – ceratobranchial 5 toothplate (7.0 mm SL) – ceratobranchial 4 (7.7 mm SL) – ceratobranchial 5 (7.8 mm SL) – ceratobranchial 1, 2 and 3, epibranchial 4 and 3, and gill rakers (8.3 mm SL) – epibranchial 2 (8.4 mm SL) – epibranchial 1 (8.9 mm SL), pharyngobranchial 3 and basibranchial 3 (9.6 mm SL) – basibranchial 2 (10.0 mm SL) – pharyngobranchial 4 (11.7 mm SL) – hypobranchial 1 (13.2 mm SL) – hypobranchial 2 (14.1 mm SL).
The sequence of ossification in the branchial skeleton differs between Noturus gyrinus and Ictalurus punctatus in that the gill rakers appear before epibranchials 1 and 2 in N. gyrinus and pharyngobranchial 4 appears after basibranchials 3 and 2. The sequence of ossification was better resolved in N. gyrinus with a general posterior to anterior direction of development in the ceratobranchials and epibranchials. Although this could not be determined in I. punctatus based on initial ossification, the same pattern could be derived from how well ossified the bones were in the earliest stages of appearance. The branchial skeleton of N. gyrinus and I. punctatus are similar, and the only difference was in the size of the gill rakers which were relatively much larger in N. gyrinus.
Weberian apparatus and associated centra
The most common sequence of ossification: centrum 4 (10.0 mm SL) – centrum 2 and 3 (10.1 mm SL) – centrum 1 (10.4 mm SL) – neural arch 4 (11.0 mm SL) – neural arch 3 (11.2 mm SL) – intercalarium, outer arm of the os suspensorium, tripus, inner arm of the os suspensorium and scaphium (12.2 mm SL) – claustrum (13.9 mm SL) (Figs
Ontogeny of the Weberian apparatus of Ictalurus punctatus in lateral view. A 10.6 mm SL. B 12.2 mm SL. C 12.7 mm SL. D 13.3 mm SL. E 15.0 mm SL. Abbreviations: Bd, Basidorsal; Cl, Claustrum; ClC, Claustrum cartilage; Exoc, Exoccipital; In, Intercalarium; NA, Neural arch; NC, Neural complex; NCC, Neural complex cartilage; IAOS, Inner arm of Os suspensorium; LP, Lateral Process; OAOS, Outer arm of Os suspensorium; Sc, Scaphium; Tr, Tripus; TS, Tectum synoticum; V, Vertebra.
Centra 1–4. Although the first four centra appear in a posterior to anterior direction starting with centrum 4, all four centra were present in some individuals of 10.0 mm SL, the smallest size in which centra were observed. All four centra originate as a pair of perichordal ossifications on the lateroventral margin of the notochord which proceed to expand and meet at the dorsal and ventral midlines. At 10.6 mm SL (Fig.
Ontogeny of the Weberian apparatus of Ictalurus punctatus in dorsal view. A 12.2 mm SL. B 12.7 mm SL. C 13.3 mm SL. D 15.0 mm SL. Abbreviations: Cl, Claustrum; In, Intercalarium; LP, Lateral Process; OAOS, Outer arm of Os suspensorium; P, Parapophysis; Ri, Rib; Sc, Scaphium; Tr, Tripus; V, Vertebra.
Ontogeny of the Weberian apparatus of Ictalurus punctatus in ventral view. A 12.2 mm SL. B 12.7 mm SL. C 13.3 mm SL. D 15.0 mm SL. Abbreviations: In, Intercalarium; IAOS, Inner arm of Os suspensorium; LP, Lateral Process; OAOS, Outer arm of Os suspensorium; P, Parapophysis; Ri, Rib; Sc, Scaphium; Tr, Tripus; V, Vertebra.
Ictalurus punctatus, Weberian apparatus of specimen TCWC 20491.06, 426 mm SL, in dorsal A, lateral B and ventral view C. Abbreviations: Cl, Claustrum; Exoc, Exoccipital; In, Intercalarium; NC, Neural complex; IAOS, Inner arm of Os suspensorium; OAOS, Outer arm of Os suspensorium; Sc, Scaphium; Tr, Tripus; V, Vertebra.
Neural Arches 3 and 4. The neural arches of vertebrae 3 and 4 first appear (10.8 mm SL) as perichondral ossification of the pair of basidorsal cartilages of centrum 3 and 4. By 12.2 mm SL (Fig.
Intercalarium. The intercalarium first appears as perichondral ossification around the basidorsal of vertebra 2 in individuals as small as 11.2 mm SL. At 12.2 mm SL (Figs
Os suspensorium. The os suspensorium first appears as a perichondral ossification around the tip of basiventral 4 (11.2 mm SL) in which the outer arm is already present as a thin anteriorly directed process. By 12.2 mm SL (Figs
Cleithral cartilage of Ictalurus punctatus (a and c, TCWC 20491.04; b, TCWC 20491.03). A Medial view of upper pectoral girdle with dotted line representing approximate location of transverse section in b; 44.9 mm SL. B Transverse section through the upper shoulder girdle; 29.0 mm SL. C Dorsal view of cleithral cartilage in situ; 16.1 mm SL. Abbreviations: Cl, Cleithrum; ClC, Cleithral cartilage; OAOS, Outer arm of Os suspensorium; Scl, Supracleithrum.
Tripus. The tripus originates as a perichondral ossification around the tip of basiventral 3 with a small membranous process directed posteriorly, the transformator process (11.2 mm SL). As the transformator process grows it curves ventromedially (12.7 mm SL; Figs
Scaphium. The scaphium appears in individuals as small as 11.9 mm SL as a perichondral ossification around the dorsal half of basidorsal 1 and a small membrane bone process can be seen extending anteriorly from the middle of the basidorsal cartilage by 12.2 mm SL (Figs
Claustrum. The claustrum is a chondral bone located between the ascending process of the scaphium and the back of the cranium. It first appears as a perichondral ossification on the anteroventral edge of the claustral cartilage, a homologue of the supradorsal cartilage of vertebra 1 (
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: centrum 1, 2, 3 and 4 (6.6. mm SL) – neural arch 4 (6.7 mm SL) – neural arch 3, outer arm of the os suspensorium, intercalarium, and tripus (7.3 mm SL) – scaphium (7.7 mm SL) – inner arm of the os suspensorium (8.7 mm SL) – claustrum (10.0 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the Weberian apparatus. The Weberian apparatus of N. gyrinus and I. punctatus are similar, and no major differences in adult morphology are observed in this region.
Post-Weberian axial skeleton
The most common sequence of ossification: centrum 5 (9.9 mm SL) – neural arch 5 (11 mm SL) – parapophyses (11.4 mm SL) – post-Weberian centra (11.7 mm SL) – post-Weberian hemal arches and post-Weberian neural arches (12 mm SL) – post-Weberian hemal spines, post-Weberian neural spines and post-Weberian ribs (12.7 mm SL) (Figs
Ontogeny of the caudal region of the axial skeleton in Ictalurus punctatus. A 10.1 mm SL. B 10.9 mm SL. C 12.2 mm SL. D 13.2 mm SL. E 15.5 mm SL. Abbreviations: Bd, Basidorsal; Bv, Basiventral; Ha, Hemal arch; Hs, Hemal spine; Na, Neural arch; Ns; Neural spine; P, Parapophysis; Ri, Rib; V, Vertebra.
Centra. The centra originate as perichordal ossifications around the notochord. Centra 5–8 are the first to ossify in the entire vertebral column in individuals as small as 9.9 mm SL. Development of the vertebral column continues anteriorly with centra 1–4 of the Weberian apparatus (see above) and posteriorly with centra 5–16 mineralized by 10.1 mm SL (Fig.
Neural arches. The neural begin as paired basidorsal cartilages along the dorsal surface of the notochord. All of the basidorsal cartilages are present by the time the first few centra are ossified. The neural arches originate as perichondral ossifications around the base of the paired basidorsal cartilages. The first neural arches originate anteriorly with basidorsals 7–14 ossifying in an individual of 10.1 mm SL. Development of the neural arches proceed bidirectionally and by 10.9 mm SL (Figs
Parapophyses. The parapophyses are chondral bones that originate from basiventral cartilages on the lateroventral surface of the abdominal centra (Fig.
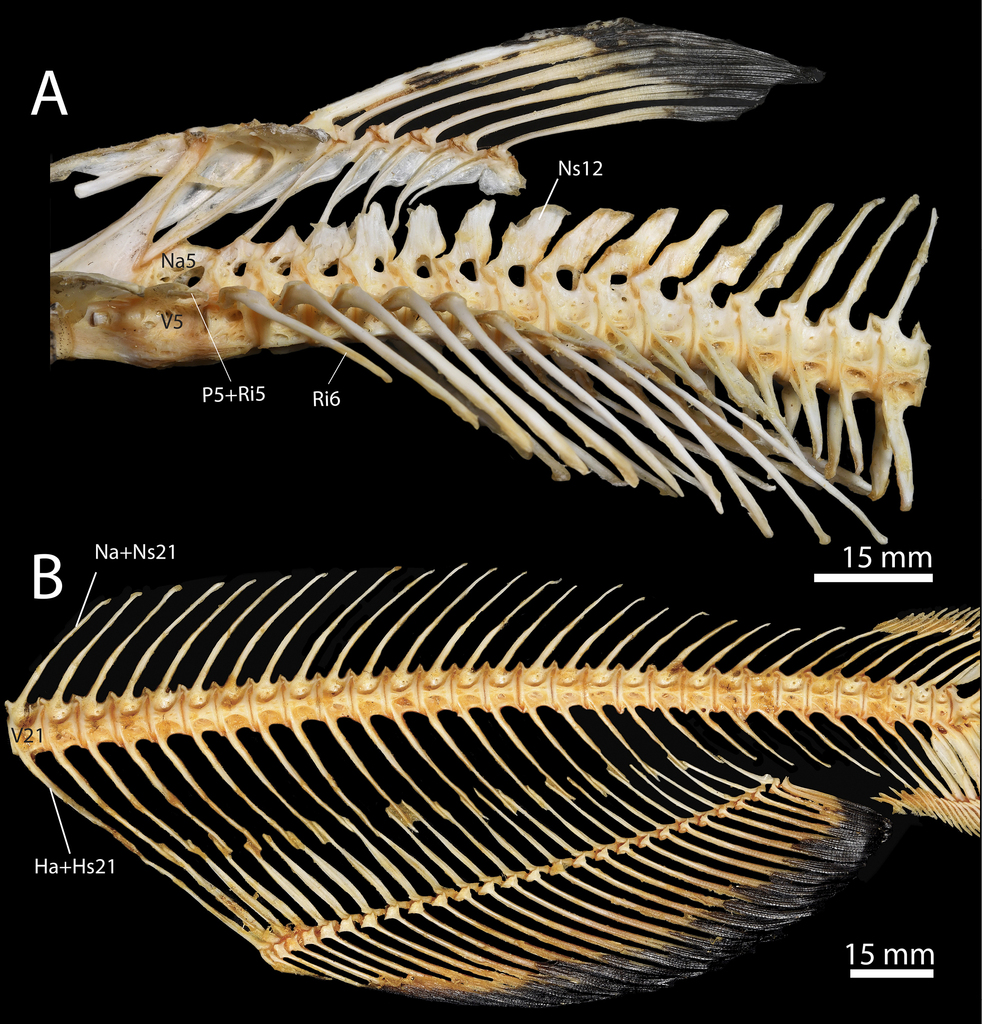
Ictalurus punctatus, specimen TCWC 20491.07, 436 mm SL (A) and TCWC 20491.08, 441 mm SL (B). A Abdominal region of the axial skeleton. B Caudal region of the axial skeleton. Abbreviations: Ha, Hemal arch; Hs, Hemal spine; Na, Neural arch; Ns; Neural spine; P, Parapophysis; Ri, Rib; V, Vertebra.
Hemal arches. The hemal arches are chondral bones that mineralize in the paired basiventral cartilages of the transitional abdominal and caudal vertebrae. Similar to the basidorsal cartilages, the basiventrals are all present by the time the earliest centra begin to ossify. They are uniform in size except for the four most posterior basiventrals which are thicker and more elongate than the preceding cartilages. The first hemal arches begin ossifying on centra 21–24, typically the first four caudal vertebrae, in individuals as small as 10.1 mm SL. Development proceeds anteriorly to the basiventrals of the transitional abdominal vertebrae which do not develop hemal spines but will meet each other across the ventral midline forming a hemal canal, and posteriorly until all of the hemal arches are perichondrally ossified (12.0 mm SL, Fig.
Hemal spines. The hemal spines originate as membrane bone except those of preural vertebrae 2–9 which are preformed in cartilage. Hemal spines were first observed ossifying anteriorly on caudal vertebrae 25–32 as small splints of membrane bone extending from the dorsal margin of the hemal arch in an individual of 11.2 mm SL. The formation of hemal spines proceeds anteriorly towards the first caudal vertebra, typically vertebra 21, and posteriorly towards the caudal skeleton with hemal spines 21–35 ossifying by 12.2 mm SL (Fig.
Neural spines. The neural spines first appear as membrane bone ossifications on the dorsal margin of the neural arches at the middle of the vertebral column (vertebrae 27–31) in individuals as small as 11.6 mm SL. Neural spines continue to develop bidirectionally with the last to ossify being those associated with preural vertebrae 2–9, whose neural spines are preformed in cartilage (Fig.
Ribs. The first ribs to ossify are ribs 6–8 in individuals as small as 12.2 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: centrum 5 and neural arch 5 (6.6 mm SL) – post-Weberian neural arches (7.2 mm SL) – post-Weberian hemal arches and parapophyses (7.3 mm SL) – post-weberian centra (7.6 mm SL) – post-Weberian ribs (8 mm SL) – post-Weberian hemal spines and post-Weberian neural spines (8.6 mm SL).
The sequence of ossification in the post-Weberian axial skeleton differs between Noturus gyrinus and Ictalurus punctatus in that the parapophyses and the post-Weberian centra complete development in N. gyrinus after the neural and hemal arches while in I. punctatus they complete development before the neural and hemal arches. The post-Weberian axial skeleton of N. gyrinus and I. punctatus are very similar, and only differ in the number of abdominal and caudal vertebrae and associated elements (typically 13 abdominal vertebrae and 28 caudal vertebrae in N. gyrinus vs. 20 abdominal vertebrae and 31 caudal vertebrae in I. punctatus).
Dorsal fin
The most common sequence of ossification: dorsal-fin spine 2 and dorsal-fin rays (11.4 mm SL) – dorsal-fin spine 1 (12.2 mm SL) – dorsal-fin proximal-middle radial 3 (12.8 mm SL) – dorsal-fin proximal-middle radial 2 and dorsal-fin proximal-middle radials (13.2 mm SL) – dorsal-fin proximal-middle radial 1 (15.0 mm SL) – dorsal-fin distal radials (15.2 mm SL). (Fig.
Dorsal-fin rays and spines. The dorsal fin, along with the anal fin, begins development after the pectoral fin. The dermal dorsal-fin rays first appear in the larval-fin fold by 11.4 mm SL. The second dorsal-fin ray, which will become dorsal-fin spine 2, is the first to appear in some individuals as small as 10.9 mm SL followed shortly by the third dorsal-fin ray (11.1 mm SL at first appearance). Development continues to proceed in a posterior direction until there is one fin ray in serial association with proximal-middle radials 2–8. At approximately the same time (12.2 mm SL; Fig.
Dorsal-fin proximal-middle radials 1, 2 and 3. Dorsal-fin proximal-middle radials 2 and 3 start off as cartilaginous rods that appear dorsal to centra 5–7 (Fig.
Dorsal-fin proximal-middle radials 4–8. The dorsal-fin proximal-middle radials originate as small cartilages in individuals as small as 10.9 mm SL (Fig.
Dorsal-fin distal radials. The dorsal-fin distal radials are chondral bones whose precursors originate as small nodules of cartilage between the proximal ends of the hemitrichia of the dorsal-fin rays (12.2 mm SL; Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: dorsal-fin spine 2 (7.2 mm SL) – dorsal-fin rays (7.3 mm SL) – dorsal-fin spine 1 (7.9 mm SL) – dorsal-fin proximal-middle radial 3 (8 mm SL) – dorsal-fin proximal-middle radial 2 (8.6 mm SL) – dorsal-fin proximal-middle radials (9 mm SL) – dorsal-fin proximal-middle radial 1 (10.2 mm SL) – dorsal-fin distal radials.
The dorsal-fin sequence of ossification was better resolved for Noturus gyrinus than Ictalurus punctatus. Dorsal-fin spine 2 ossifies before the remaining dorsal-fin rays in N. gyrinus while in I. punctatus they have the same fixed length. However, some individuals of I. punctatus did possess dorsal-fin spine 2 before the remaining dorsal-fin rays suggesting the same pattern of appearance. Additionally, dorsal-fin proximal-middle radial 2 ossifies before the remaining proximal-middle radials in N. gyrinus while in I. punctatus they appeared at the same time. The dorsal fin of N. gyrinus is very similar to that of I. punctatus with the only noted difference being the shape of dorsal-fin spine 2 which lacks anterior denticuli and posterior serrations in N. gyrinus.
Anal fin
The most common sequence of ossification: anal-fin rays (11.4 mm SL) – anal-fin proximal-middle radials (13.4 mm SL) – anal-fin distal radials (21.2 mm SL). (Fig.
Anal-fin rays. The anal-fin develops at approximately the same time as the dorsal fin with the first fin rays appearing in individuals as small as 10.9 mm SL. By 11.7 mm SL (Fig.
Anal-fin proximal-middle radials. The precursors of anal-fin proximal-middle radials first appear as small nodules of cartilage along the posteroventral midline of the body in some individuals as small as 10.1 mm SL (Fig.
Anal-fin distal radials. The anal-fin distal radials first appear as small round cartilages between the proximal bases of the anal-fin ray hemitrichia in individuals as small as 12.2 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: anal-fin rays (7.3 mm SL) – anal-fin proximal-middle radials (8.9 mm SL) – anal-fin distal radials.
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the anal fin. Only two differences were observed between N. gyrinus and I. punctatus. The first is that the anal-fin formula of N. gyrinus is iv.11–iv.13 compared to I. punctatus in which it is v.22–v.24. Additionally, N. gyrinus possesses a distal radial cartilage in serial association with the anteriormost proximal-middle radial unlike that of I. punctatus.
Caudal fin and supporting skeleton
The most common sequence of ossification: principal caudal-fin rays (8.7 mm NL) – ventral procurrent caudal-fin rays (10.9 mm SL) – anterior ural centrum and preural centrum 2 and 3 (11.7 mm SL) – posterior ural centrum and uroneural 1 (11.9 mm SL) – hemal arch of PU2 and PU3, hypurals 1, 2, 3, and 4, neural arch of PU2 and PU3 and parhypural (12 mm SL) – hemal spine of PU2 and PU3 (12.2 mm SL) – hypural 5 and dorsal procurrent caudal-fin rays (12.5 mm SL) – neural spine PU3 (12.7 mm SL) – hypural 6 (12.8 mm SL) – epural (13.4 mm SL) (Fig.
Ontogeny of the caudal-fin skeleton in Ictalurus punctatus. A 8.9 mm NL. B 9.7 mm NL. C 11.6 mm SL. D 11.9 mm SL. E 12.3 mm SL. F 15.0 mm SL. Abbreviations: AC, Anterior ural centrum; Bd, Basidorsal; Bv, Basiventral; Ep, Epural; EpC, Epural cartilage; H, Hypural; Ha, Hemal arch; HC, Hypural cartilage; Hs, Hemal spine; HsC; Hemal spine cartilage; Na, Neural arch; Ns, Neural spine; NsC, Neural spine cartilage; PC, Posterior ural centrum; Ph, Parhypural; PhC, Parhypural cartilage; PU, Preural centrum; R, Caudal-fin ray; Un, Uroneural.
Principal caudal-fin rays. The first principal caudal-fin rays to form are associated with hypural cartilages 2 and 3 at 8.7 mm NL (Fig.
Procurrent caudal-fin rays. The ventral procurrent rays are the first to appear with a single procurrent ray forming anterior to the ventralmost principal caudal-fin ray at 10.9 mm SL. The ventral procurrent rays are added anteriorly with two additional rays ossifying by 11.9 mm SL (Fig.
Anterior and posterior ural centra. The anterior ural centrum appears as a perichordal ossification of the notochord along the bases of the parhypural and hypurals 1 and 2 in individuals as small as 10.9 mm SL (fixed in development at 11.7 mm SL). The perichordal ossification expands dorsolaterally until the two sides meet at the distal midline forming a complete ring of bone around the notochord (11.6 mm SL; Fig.
Ictalurus punctatus, specimen TCWC 20491.06, 426 mm SL (A), (B), (C), and TCWC 20491.07, 436 mm SL (D) and (E). A Dermal pectoral girdle in lateral view B Endoskeletal pectoral girdle in medial view C Pectoral girdle in dorsal view D Caudal skeleton in lateral view E Pelvic-girdle in ventral view. Abbreviations: AC, Anterior ural centrum; Bp, Basipterygium; Cl, Cleithrum; Co, coracoid; Ep, Epural; H, Hypural; Ha, Hemal arch; Hp, Humeral process; Hs, hemal spine; IsPr, Ischiac process; MscA, Mesocoracoid arch; Na, Neural arch; Ns, Neural spine; PC, Posterior ural centrum; PcSp, Pectoral-fin spine; Ph, Parhypural, Pl, Pleurostyle; PlvSpl, Pelvic splint; PU, Preural centrum; R, Soft fin ray; Scl, Supracleithrum; SF, Scapular foramen; SpF, Spinal fossa; U, Ural centrum.
Preural centra 2 and 3. Preural centra 2 and 3 are the last centra in the vertebral column to ossify and first appear in individuals of 11.0 mm SL and their presence is fixed in development at 11.7 mm SL. Both centra originate as four separate perichordal ossifications around the base of the basidorsal and basiventral cartilages. Unlike preceding centra, the ossifications of PU2 and PU3 meet across the dorsal and ventral midline of the notochord first (11.9 mm SL; Fig.
Uroneural 1. The single pair of uroneurals originates as thin paired splints of membrane bone dorsolateral to the anterior ural centrum in individuals as small as 11.2 mm SL (Fig.
Hemal arches and spines of preural centra 2 and 3. The hemal arches of PU2 and PU3 are chondral bones that form from the basiventral cartilages of their respective centra. The basiventral of PU2 is larger and develops faster than the other basiventral cartilages. By 9.7 mm NL (Fig.
Hypurals 1–6. The six hypurals are preformed in cartilage along the ventral end of the notochord. The cartilaginous precursors of hypurals 1 and 2 are the first skeletal elements to appear in the axial skeleton and were observed in the smallest specimen examined (7.7 mm NL). Hypural cartilage 3 appears at approximately the same time as the first caudal fin-rays are beginning to form (8.6 mm NL) as the tip of the notochord is just starting to curve dorsally. By 9.7 mm NL (Fig.
Neural arches and spines of preural centra 2 and 3. The neural arches of PU2 and PU3 begin development as basidorsal cartilages associated with their respective centra (9.7 mm NL; Fig.
Parhypural. The parhypural starts as a pair of cartilages shortly after the appearance of hypural 1 and 2 cartilages (8.7 mm NL; Fig.
Epural. The epural is a chondral bone and the last skeletal element in the caudal skeleton to ossify. It first originates from a small cartilage which can be seen in individuals of 11.6 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: principal caudal-fin rays (5.9 mm SL) – hypural 1 and 2 and anterior ural centrum (7 mm SL) – parhypural, dorsal and ventral procurrent caudal-fin rays (7.2 mm SL) – neural arch PU3, hypural 3 and posterior ural centrum (7.3 mm SL) – preural centrum 3 (7.6 mm SL) – hemal arch PU2 and PU3, hemal spine PU2 and PU3, neural arch PU2 and hypural 4 (7.7 mm SL) – uroneural 1 (7.9 mm SL) – preural centrum 2 (8.3 mm SL) – neural spine PU3 (8.6 mm SL) – neural spine PU2 and hypural 5 (8.7 mm SL) – epural (9.8 mm SL)
The sequence of development of Noturus gyrinus has a two differences when compared to Ictalurus punctatus. The first difference is that the dorsal and ventral procurrent caudal-fin rays start to ossify at the same time in N. gyrinus while in I. punctatus the dorsal procurrent fin-rays start to ossify much later after most of the other elements of the caudal skeleton. The second major difference is that preural centra 2 and 3 appear later in development in N. gyrinus, not ossifying until after the two ural centra, the parhypural and hypurals 1–3 while in I. punctatus they are some of the first elements to ossify in the region at approximately the same time as the ural centra and earlier than the parhypural and hypurals. The caudal skeleton of N. gyrinus and I. punctatus, despite differing in their external appearance, are similar internally, with the only differences being in the total number of hypurals (5 in N. gyrinus vs. 6 in I. punctatus) and that there is not a well-developed shelf of bone for the origin of the hypochordal longitudinal muscle.
Pectoral girdle
The most common sequence of ossification: cleithrum (7.7 mm NL) – pectoral-fin rays (10.9 mm SL) – supracleithrum (11.4 mm SL) – coracoid (13.2 mm SL) – propterygium (13.9 mm SL) – pectoral-fin radial 3 and 4 (14.2 mm SL) (Fig.
Ontogeny of the dermal pectoral girdle of Ictalurus punctatus. A 10.0 mm NL. B 11.8 mm SL. C 13.3 mm SL. D 15.0 mm SL. E 44.9 mm SL. Abbreviations: Co, Coracoid; Cl, Cleithrum; Hp, Humeral process; Pcl, Postcleithrum; PcRC, Pectoral radial cartilage; PcSp, Pectoral-fin spine; R, Pectoral-fin ray; ScCoC, Scapulocoracoid cartilage; Scl, Supracleithrum.
Cleithrum. The dermal cleithrum appears in individuals as small as 7.7 mm SL. It starts off as a slightly curved, thin split of bone just posterior to the cranium and anterodorsal to the yolk-sac. By 10.0 mm SL (Fig.
Pectoral-Fin Rays. The pectoral fin is the second fin to develop in I. punctatus with the first two fin rays appearing at 10.9 mm SL. The anteriormost pectoral-fin ray which will become the pectoral-fin spine is approximately twice the size of subsequent fin rays and its dorsal hemitrichium is closely associated with the propterygium. Four more fin-rays have appeared by 12.0 mm SL and the first four are now segmented. The dorsal and ventral hemitrichia of the first segment of the anteriormost fin ray have started to fuse across the anterior edge and eight fin rays (including the spine) have formed. A second segment has fused to the spine proper and the propterygium has perichondrally ossified and is fused to the proximal head of the upper hemitrichium of the spine at 14.1 mm SL. At approximately the same time as a third segment is added to the spine proper (15.0 mm SL; Fig.
Supracleithrum. Whether this element is of compound origin (posttemporal+supracleithrum) or not has been a contentious subject in the past. Herein I refer to the element as the supracleithrum and further discuss the homology of this element below (see discussion). The dermal supracleithrum first appears as a thin splint of bone anterolateral to the dorsal tip of the cleithrum (10.9 mm SL; Fig.
Coracoid. Whether this element is of compound origin (scapula+coracoid) or not has been previously undetermined. Herein I refer to the element as the coracoid and further discuss the homology of this bone in the discussion. The coracoid first appears as a perichondral ossification around the proximal end of the ventral arm of the scapulocoracoid cartilage (12.6 mm SL). By 13.1 mm SL (Fig.
Ontogeny of the endoskeletal pectoral girdle (A-C, scapulocoracoid; D-G, pectoral radials) of Ictalurus punctatus A 13.3 mm SL. B 15.0 mm SL. C 44.9 mm SL. D 10.0 mm NL. E 10.8 mm SL. F 12.9 mm SL. G 44.9 mm SL. Asterisk indicates abductor coracoid lamina. Black arrows indicates scapular process of Coracoid. Abbreviations: Co, Coracoid; Cl, Cleithrum; cPDRC, complex pectoral distal radial cartilage; DRC, distal radial cartilage; Hp, Humeral process; MscA, Mesocoracoid arch; PcSp, Pectoral-fin spine; PR, Pectoral radial; PRC, Pectoral radial cartilage; Ptg, Propterygium; PtgC, Propterygial cartilage; ScCoC, Scapulocoracoid cartilage; SF, Scapular foramen; SpF, Spinal fossa.
Pectoral-Fin Radials. Two small pectoral radial cartilages are present by 10.0 mm SL (Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: cleithrum (5.4 mm NL) – pectoral-fin rays (6.4 mm SL) – supracleithrum (7.0 mm SL) – coracoid (8.3 mm SL) – propterygium (9.9 mm SL) – pectoral-fin radial 3+4 (11.1 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the pectoral girdle. The pectoral girdle of N. gyrinus and I. punctatus are similar, except for the shape of the pectoral-fin spine, which lacks posterior serrations and anterior denticuli in N. gyrinus, and the pectoral-fin radials, in which there is only a single pectoral-radial element in N. gyrinus which is a compound element consisting of pectoral radials 3 and 4.
Pelvic girdle
The most common sequence of ossification: pelvic-fin rays (12.3 mm SL) – basipterygium (15.0 mm SL) (Fig.
Pelvic-Fin Rays. The pelvic fin is the last of the fins to develop with fin rays first appearing in individuals of 11.9 mm SL on the posterolateral margin of the yolk-sac. By 12.6 mm SL, resorption of the yolk-sac has resulted in the fins sitting in their normal position on the ventral margin of the body anterior to the anus. At this size, five small fin rays are present but remain unsegmented. At 14.1 mm SL, the number of pelvic-fin rays equals that of adults (i.7) and all eight fin rays are segmented. A small pelvic splint (not included in the ossification sequence) has appeared on the lateral margin of the fin by 15.9 mm SL.
Basipterygium. The basipterygium starts as a perichondral ossification of the basipterygial cartilage between the anterior foramen and the anterior edge of the cartilage (14.5 mm SL; Fig.
Comparison with Noturus gyrinus. The most common sequence of ossification for this region in Noturus gyrinus is as follows: pelvic-fin rays (8.3 mm SL) – basypterygium (10.4 mm SL).
No differences in the sequence of ossification were identified between Noturus gyrinus and Ictalurus punctatus in the pelvic girdle. The pelvic girdle of N. gyrinus and I. punctatus are similar, except for the ischiac processes which are much shorter and the outer edge of the basipterygia which reaches approximately half the distance of the lateral anterior process in N. gyrinus.
Discussion
Skeletal development in Ictalurus punctatus and Noturus gyrinus
The development of the skeleton in Ictalurus punctatus and Noturus gyrinus occurred over a relatively short period of growth, with all elements of the skeleton (excluding the dorsal- and anal-fin distal radials in N. gyrinus) present by 22.4 and 14.1 mm SL, respectively. Dorsal- and anal-fin distal radials, which are present in the adult stage of N. gyrinus (41.5 mm SL, TCWC 15438.13), are absent from the developmental series compiled for this study suggesting that these elements form later in development, at sizes larger than that of the material examined herein (max size 26.4 mm SL). Elements of the skeleton typically appeared at smaller sizes in N. gyrinus compared to I. punctatus, which is not surprising given that the former is much smaller than the latter and it is generally observed that smaller bodied species develop faster than closely related, larger bodied species (
Intraspecific variation in Ictalurus punctatus and Noturus gyrinus
Low levels of intraspecific variation in the total number of certain serial elements were observed in both species. In I. punctatus, having 11 post-Weberian ribs associated with vertebrae 5–15 was the most common condition although in a small number of individuals (n=11) an additional 12th rib associated with vertebra 16 was observed. The majority of individuals examined had 20 abdominal vertebrae and 31 caudal vertebrae although individuals with 19 abdominal centra and 30 or 32 caudal centra were observed, resulting in a total vertebral count ranging from 49–52. Variation in the presence of a neural spine on PU2 was also observed. The majority of individuals possess a neural spine associated with PU2 (Fig.
Comparison of skeletal development with other Otophysans
Although there have been numerous studies of skeletal development in otophysans, most of these have focused only on a subsection of the skeleton (e.g., cranium and paired fins, post-cranial skeleton, Weberian apparatus;
Danio rerio and Enteromius holotaenia. In the ethmoid region, the nasal is the first bone to appear in Ictalurus punctatus and appears much earlier in the entire sequence of ossification compared to D. rerio and E. holotaenia, in which it does not appear until much later in development and is the last bone in the region to appear. The lateral ethmoid appears later in I. punctatus, being the third bone to appear in the region, while it is the first bone to appear in the ethmoid region of D. rerio and is present much earlier in the entire sequence of ossification. E. holotaenia does not exhibit this shift in lateral ethmoid development and instead, the first bone of the ethmoid region to appear is the vomer compared to it being the last bone to appear in this region in I. punctatus. In the otic region, the dermopterotic is the second element of the region to appear in I. punctatus, after the prootic and before the autopterotic and autosphenotic. In the cypriniforms, the dermopterotic is the last element to appear in the region and is one of the last to appear in the overall sequence of ossification.
In the suspensorium of Ictalurus punctatus, both the hyomandibular and endopterygoid appear much later in the regional sequence as well as the entire ossification sequence when compared to that of Danio rerio, in which both ossifications represent some of the first bones to appear in the sequence of ossification. Unfortunately, in Enteromius holotaenia the smallest individual observed had already 14 bones which included many of the elements of the hyopalatine arch, jaws, and opercular series so it is not possible to compare the sequence of development in most of this region. Among the elements of the branchial arches, ceratobranchial 5 ossifies later in I. punctatus, being the 62nd element to ossifiy while in the cypriniforms it is one of the first bones to appear. In I. punctatus, basibranchials 2 and 3 are among the last elements of the skeleton with the hypobranchials being the only other elements of the branchial arches to ossify later. In E. holotaenia the basibranchials appear much earlier in the sequence and before several other branchial arch elements. The sequence of ossification of the elements of the hyoid bar of I. punctatus differ from E. holotaenia in the earlier appearance of the urohyal and interhyal and the later appearance of the dorsal hypohyal. The urohyal is the third element of the region to appear in I. punctatus while it is the second to last to appear in E. holotaenia. The interhyal appears at approximately the same time as the posterior ceratohyal in I. punctatus but well after all other elements of the hyoid bar in E. holotaenia. The dorsal hypohyal is one of the last elements to appear in I. punctatus while in E. holotaenia it ossifies before approximately two-thirds of the skeleton.
In the post-cranial skeleton, the pectoral- and dorsal-fin rays appear early in the ossification sequence of Ictalurus punctatus while in the cypriniforms they ossify much later in the entire sequence of ossification. In the case of the procurrent caudal-fin rays, the ventral-procurrent caudal-fin rays of I. punctatus appear very early in the sequence of ossification while the dorsal-procurrent caudal-fin rays appear much later in the overall sequence. In Danio rerio and Enteromius holotaenia, both the dorsal and ventral procurrent caudal-fin rays appear at approximately the same time in the middle of the sequence. It is also worth noting that in I. punctatus the fin rays associated with all fins (except for the pelvic fin) ossify before the endoskeletal elements of the caudal fin, while in the cypriniforms most of the caudal skeleton is ossified before any of the other fin rays start to develop. In the vertebral column the most notable difference between I. punctatus and the cypriniforms is the early appearance of the parapophyses, which start to ossify before all of the post-Weberian centra in I. punctatus, but do not start to ossify until almost the entire vertebral column (excluding some of the hemal and neural spines and ural centrum 2) has formed in D. rerio and E. holotaenia. The outer arm of the os suspensorium is the second of the Weberian elements to ossify in I. puncatutus while in D. rerio and E. holotaenia it is the second to last, just before the claustrum. Additionally, the intercalarium, inner arm of the os suspensorium, scaphium and tripus all start to ossify slightly later in the overall sequence of I. punctatus when compared to that of the two cypriniforms. The centra associated with the caudal skeleton (preural centra 2 and 3 and the two ural centra) appeared earlier in the sequence of ossification of I. punctatus before the Weberian ossicles and some of the hemal and neural arches; in E. holotaenia these centra were some of the last elements of the vertebral column to ossify.
Salminus brasiliensis. In the cranial skeleton, the nasal and dermopterotic appeared much earlier in the regional sequences of ossification as well as that of the whole skeleton in Ictalurus punctatus compared to S. brasiliensis. Likewise, the hyomandibular and endopterygoid appeared much later in I. punctatus while both appeared very early in the sequence of S. brasiliensis. The gill rakers of I. punctatus do not appear until after all of the ceratobranchials and epibranchials are ossified while in S. brasiliensis the gill rakers ossified very early in development, before ceratobranchial 4 or any of the epibranchials had appeared. Additionally, the interhyal in I. punctatus appears at the same time as the posterior ceratohyal and before the dorsal hypohyal. This is slightly earlier in the overall sequence compared to S. brasiliensis in which it forms towards the end of the sequence, well after the other elements of the hyoid bar had ossified.
In the postcranial skeleton, the pectoral-fin rays and the parapophyses showed the same pattern of appearing much earlier in the sequence of Ictalurus punctatus when compared to Salminus brasiliensis. The ventral procurrent caudal-fin rays appear much earlier in I. punctatus while the dorsal procurrent caudal-fin rays appear at roughly the same place in the sequence as S. brasiliensis. Neural arches 3 and 4 also appear much earlier in the sequence of I. punctatus, ossifiying before most of elements of the vertebral column while in S. brasiliensis they do not ossify until after most of the vertebral column is present. Finally, like what was observed in the comparison with E. holotaenia, the four centra supporting the caudal skeleton appear much earlier in the sequence of ossification of I. punctatus while in S. brasiliensis they were some of the last elements of the vertebral column to appear.
Differences shared between Danio rerio, Enteromius holotaenia and Salminus brasiliensis. The relative timing of appearance for bony skeletal elements varied between Ictalurus punctatus and the three non-siluriform otophysans. Most of this variation was restricted to only slight shifts in the relative position of skeletal elements in comparisons of either regional or the entire sequences. However, several major differences were observed between the ossification sequence compiled for I. punctatus and those for all three non-siluriform otophysan species, several of which were consistent across all three comparisons. In the neurocranium, elements associated with the cephalic lateral line canals (e.g., nasal, dermopterotic) all appear much earlier in the development of I. punctatus. This also applies to the lacrimal, another element associated with the cephalic lateral line sensory system, which is one of the first elements to appear in I. punctatus but does not make an appearance until much later in development in the three other species of otophysans. The hyomandibular and endopterygoid also appear later in the sequence of ossification of I. punctatus.
In the post-cranial skeleton, the pectoral-fin rays, ventral procurrent caudal-fin rays, and the parapophyses all appear very early in the sequence of ossification compiled for Ictalurus compared to the three non-siluriform otophysans. Of particular interest is the much earlier appearance of the pectoral-fin rays in the two ictalurids, which are the 21st or the 11th bones to appear in the sequence of I. punctatus and Noturus gyrinus, respectively, versus the 100th to 125th in the three non-siluriform otophysans. The anteriormost pectoral-fin ray of most catfishes is modified into a robust lockable spine that exhibits a remarkable amount of morphological variation (e.g.,
Although the aforementioned differences in the appearance of ossifications were consistently observed in comparisons between Ictalurus punctatus and the three non-siluriform otophysans, it is not possible to tell whether these consistent patterns are due to chance, as variation exists throughout the entire ossification sequence of these taxa, or if these differences in the timing of appearance of these elements could be derived characters of ictalurids or even Siluriformes. In other groups of vertebrates (e.g., amphibians, squamates, birds, and mammals;
Homology
Supraoccipital. In the posterodorsal surface of the neurocranium, teleosts typically possess a pair of parietals, dermal bones which form via intramembranous ossification in the dermis, and a single median supraoccipital, a chondral bone which ossifies around and replaces a portion of the otic capsule cartilage. Unlike teleosts, siluriforms only possess a single median bone in this region which is hypothesized to result from the fusion of the parietals and the supraoccipital (
At no point in the development of the supraoccipital in Ictalurus punctatus and Noturus gyrinus was fusion between dermal and chondral ossifications observed (see description of supraoccipital development in I. punctatus; Fig.
Supracleithrum. In teleosts, the dermal component of the pectoral-girdle is typically comprised of three elements (cleithrum, supracleithrum, and posttemporal). Siluriformes differ from this condition in that they possess only two major dermal bones in the pectoral-girdle, the cleithrum and an upper element of the pectoral girdle which has previously been interpreted as: (1) the posttemporal (
In the developmental series of the two species of ictalurids examined herein, the upper element of the pectoral-girdle originates from a single ossification that forms lateral to the dorsal tip of the cleithrum and no evidence of fusion during the ontogeny of this element was observed (Fig.
Posttemporal and Extrascapular. In teleosts, the posterior portion of the cranium that connects to the pectoral girdle typically possesses an extrascapular which carries the sensory canal from the posttemporal to the neurocranium. Catfishes, unlike most teleosts, exhibit variation in the number, shape and relationship of bones in this region (summarized in Table
Summary of diversity in the elements of the cranio-pectoral articulation of catfish species examined.
| Family | Species | Cranio-Pectoral Articulation |
|---|---|---|
| Loricarioidea | ||
| Astroblepidae | Astroblepus sp. | Posttemporal absent; Supracleithrum attached directly to the pterotic. |
| Callichthyidae | Megalechis thoracata | Posttemporal absent; Supracleithrum attached directly to the pterotic. |
| Dianema longibarbis | Posttemporal absent; Supracleithrum attached directly to the pterotic. | |
| Corydoras melini | Posttemporal absent; Supracleithrum attached directly to the pterotic. | |
| Corydoras panda | Posttemporal absent; Supracleithrum attached directly to the pterotic. | |
| Loricariidae | Ancistrus sp. | Posttemporal absent; Supracleithrum attached directly to the pterotic. |
| Pareiorhaphis vestigipinnis | Posttemporal absent; Supracleithrum attached directly to the pterotic. | |
| Nematogenyidae | Nematogenys inermis | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Scoloplacidae | Scoloplax empousa | Posttemporal absent; Supracleithrum attached directly to the pterotic. |
| Trichomycteridae | Henonemus sp. | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Trichomycterus punctulatus | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Potamoglanis hasemani | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Diplomystoidea | ||
| Diplomystidae | Diplomystes chilensis | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Siluroidea | ||
| Amblycipitidae | Amblyceps cerinum | Posttemporal present as a simple canal ossification between the supracleithrum and pterotic. |
| Amblyceps mangois | Posttemporal present as a simple canal ossification between the supracleithrum and pterotic. | |
| Liobagrus somjinensis | Posttemporal present as a simple canal ossification between the supracleithrum and pterotic. | |
| Amphiliidae | Amphilius uranoscopus | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Anchariidae | Ancharius fuscus | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Ariidae | Ariopsis felis | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Ariopsis seemanni | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. | |
| Bagre marinus | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. | |
| Aspredinidae | Bunocephalus sp. | Posttemporal present as a simple canal ossification between the supracleithrum and pterotic. |
| Pseudobunocephalus lundbergi | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Auchenipteridae | Tatia intermedia | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Trachycorystes sp. | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Auchenoglanididae | Auchenoglanis occidentalis | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Austroglanididae | Austroglanis gilli | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Bagridae | Pseudomystus siamensis | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Cetopsidae | Cetopsis coecutiens | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Helogenes marmoratus | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Chacidae | Chaca chaca | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Clariidae | Clarias batrachus | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Clarias gariepinus | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Claroteidae | Chrysichthys mabusi | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Cranoglanididae | Cranoglanis bouderius | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Doradidae | Ossancora punctata | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Platydoras armatulus | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Heptapteridae | Goeldiella eques | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Heteropneustidae | Heteropneustes fossilis | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Horabagridae | Horabagrus brachysoma | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Ictaluridae | Ameiurus melas | Posttemporal and Accessory Posttemporal present between supracleithrum and pterotic. |
| Ictalurus furcatus | Posttemporal and Accessory Posttemporal present between supracleithrum and pterotic. | |
| Ictalurus punctatus | Posttemporal and Accessory Posttemporal present between supracleithrum and pterotic. | |
| Noturus flavus | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Noturus gyrinus | Posttemporal present as a simple canal ossification between the supracleithrum and pterotic. | |
| Pylodictis olivaris | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. | |
| Kryptoglanididae | Kryptoglanis shajii | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Malapteruridae | Malapterurus oguensis | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Mochokidae | Chiloglanis sp. | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Euchilichthys sp. | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Microsynodontis sp. | Posttemporal absent; Supracleithrum forms part of skull roof. | |
| Pangasiidae | Pangasius macronema | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Pimelodidae | Pimelodus pictus | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Plotosidae | Plotosus lineatus | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Pseudopimelodidae | Microglanis cf. iheringi | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Microglanis poecilus | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. | |
| Ritidae | Rita rita | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. |
| Schilbeidae | Parailia congica | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| Parailia pellucida | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Schilbe intermedius | Posttemporal present as a plate-like bone between the supracleithrum and pterotic. | |
| Siluridae | Silurus asotus | Posttemporal absent; Supracleithrum articulates directly with the pterotic. |
| S. glanis | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Wallago attu | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Sisoridae | Glyptothorax lampris | Posttemporal absent; Supracleithrum forms part of skull roof. |
| Parachiloglanis | Posttemporal absent; Supracleithrum articulates directly with the pterotic. | |
| Pseudolaguvia kapuri | Posttemporal absent; Supracleithrum forms part of skull roof. | |
Skeletal elements associated with the articulation of the cranium and pectoral girdle. A Diplomystes papillosus, CAS 81539, 118 mm SL. B Cranoglanis bouderius, CAS 69758, 97.0 mm SL. Arrows indicate anterodorsal tip of supracleithrum. Abbreviations: Asph, AutosphenoticEpoc, Epioccipital; Pt, Posttemporal; Pto, Pterotic; Scl, Supracleithrum; Soc, Supraoccipital.
To date, there are three hypotheses of the homology of elements in this region: (1) the plate-like bone represents the posttemporal and the extrascapular is lost (
In the 21 species of catfishes examined in the present study that possess the plate-like element, the bone always shares the same relationship with the surrounding elements of the neurocranium. The bone is sutured to the pterotic and the supraoccipital anterolaterally and anteromedially, respectively. Posteriorly, it overlays and forms a firm connection to the anterodorsal tip of the supracleithrum via dense connective tissue (Fig.
Neither Ictalurus punctatus or Noturus gyrinus possess the plesiomorphic condition described above for catfishes; however, the single plate-like posttemporal likely represents the plesiomorphic condition of the family as this condition is also exhibited by the Cranoglanididae (Fig.
Ontogeny of the cranial bones associated with the articulation of the pectoral girdle of Ictalurus punctatus. A 15.4 mm SL. B 44.9 mm SL. C 441 mm SL. Abbreviations: *, Accessory posttemporal; Asph, Autosphenotic; Epoc, Epioccipital; Pt, Posttemporal; Pto, Pterotic; Scl, Supracleithrum; Soc, Supraoccipital.
In the developmental series of Ictalurus punctatus examined herein, the canal bearing element is the first of the two elements to appear (~12.8 mm SL; Figs
Neuromasts and associated cranial bones of select catfishes Pimelodus pictus (TCWC 20491.12, 42.0 mm SL; A,E), Pylodictis olivaris (TCWC 20490.04, 56.0 mm SL; B, F), Ictalurus punctatus (TCWC 20490.02, 60.0 mm SL; C, G) and Noturus gyrinus (TCWC 20490.03, 27.0 mm SL; D, H). A–D Cranial bones stained with alizarin red S. White dots correspond to canal neuromasts of E–H. E–H Canal neuromasts stained using 4-Di-2-ASP following
Based on the developmental series of Ictalurus punctatus examined herein, in addition to the association and location of the two elements in adult specimens, I narrow down the homology of these bones to two hypotheses: (1) the canal bearing element is homologous to the posttemporal and the additional plate-like bone represents a de novo ossification; or (2) the single posttemporal present in the plesiomorphic condition has become fragmented and is represented by two separate ossifications. Regardless of the hypothesis, the canal bearing element is interpreted as either the whole or part of the posttemporal due to it carrying the same sensory canal ossification as that observed in the single plate-like posttemporal of the plesiomorphic condition. This conclusion is in agreement with those of both
Finally, it is worth mentioning that the examination of the canal neuromasts in this region of select catfishes (Fig.
In ictalurids in which two elements are present in this region,
Coracoid. In teleosts, the scapulocoracoid cartilage typically gives rise to three separate ossifications, the dorsal scapula, ventral coracoid and medial mesocoracoid. The single endoskeletal element in the pectoral-girdle of catfishes has previously been assumed to represent the product of ontogenetic fusion between the three endochondral ossifications, based on comparisons of the adult skeleton (
Dorsal-fin Spine 2. The soft dorsal-fin rays of teleosts typically consist of two separate segmented hemitrichia, the proximal bases of which articulate directly with the lateral surface of the distal radial that is in serial association with that fin ray. In many catfishes, the hemitrichia and developing distal segments of the second dorsal-fin ray fuse into a lockable dorsal-fin spine. The proximal base halves of this dorsal-fin spine are also fused across the midline, and rather than articulating with a typical distal radial, form a foramen through which an ossified ring-like process of proximal-middle radial 3 passes, establishing in the dorsal-fin spine a “chain-link” articulation (sensu
Urohyal. The urohyal of teleosts is a median bone that originates in the tendons of the sternohyoideus muscle as a single ossification. The urohyal of catfishes differs from that of other teleosts in that it originates as two separate ossification centers, which fuse during development into a single element (
Lacrimal. The infraorbital elements of teleosts are typically comprised of plates of dermal bone that carry the infraorbital sensory canal. In catfishes, the infraorbitals differ from the typical condition in that they are greatly reduced, represented by only simple tube-like ossifications around the infraorbital canal, except for the anteriormost infraorbital element. This bone has a laminar portion that carries the ossified canal. Homology of this anteriormost element still remains uncertain. Previous studies have referred to it as either infraorbital 1, or the lacrimal (
Acknowledgements
This study represents part of my doctoral dissertation conducted at Texas A&M University, under the direction of K. W. Conway. I would like to thank all members of my dissertation committee (including K. O. Winemiller, G. Voelker, C. D. Marshall, and R. Britz) for their helpful advice and comments which have greatly improved the contents of this dissertation. I am grateful to B. Brown (AMNH), M. Sabaj (ANSP), O. Crimmen (BMNH), D. Catania (CAS), C. Dillman (CUMV), C. McMahan (FMNH), A. Bentley (KU), R. Feeney (LACM), H. Prestridge (TCWC), P. Harris (UAIC), D. Nelson (UMMZ), and J. Williams (USNM) for the loan of specimens and/or assistance with specimen curation. I also thank D. Gatlin III and B. Ray for providing access to eggs of Ictalurus punctatus, R. O’Hanlon, C. Mynatt, K. W. Conway, and A. K. Pinion for help in the collection of Noturus gyrinus. Additional thanks to K. W. Conway for help with histological sectioning, neuromast staining, and photography of skeletal preparations. I would also like to thank G. Mattox for critical comments and J. Lundberg for discussions that helped to improve the quality of this manuscript. Finally, I thank K. W. Conway and R. Britz for critical advice and countless discussions concerning all aspects of this study. This research was possible with funding from Texas A&M Agrilife Research (TEX09452 to K. W. Conway) and represents publication number 1664 of the TAMU BRTC and publication 08 of the TAMU Aquarium Research Facility.
References
- Adriaens D, Verraes W (1998) Ontogeny of the osteocranium in the African catfish, Clarias gariepinus Burchell (1822) (Siluriformes: Clariidae): ossification sequence as a response to functional demands. Journal of Morphology 235: 183–237. https://doi.org/10.1002/(SICI)1097-4687(199803)235:3<183::AID-JMOR2>3.0.CO;2-8
- Albert JS (2001) Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Miscellaneous Publications of the Museum of Zoology University Michigan 190: 1–127.
- Alexander RMcN (1966) Structure and function in the catfish. Journal of Zoology 148: 88–152. https://doi.org/10.1111/j.1469-7998.1966.tb02943.x
- Allis EP (1904) The latero-sensory canals and related bones in fishes. Internationale Monatsshrift für Anatomie und Physiologie 21: 401–503.
- Arratia G (1987) Description of the primitive family Diplomystidae (Siluriformes, Teleostei, Pisces): morphology, taxonomy and phylogenetic implications. Bonner Zoologische Monographien 24: 1–120
- Arratia G (1992) Development and variation of the suspensorium of primitive Catfishes (Teleostei: Ostariophysi) and their phylogenetic relationships. Bonner Zoologische Monographien 32: 1–149.
- Arratia G (2003a) Catfish head skeleton—an overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R (Eds) Catfishes, vol. 1. Science Publishers, Enfield, 3–46.
- Arratia G (2003b) The siluriform postcranial skeleton—an overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R (Eds) Catfishes, vol. 1. Science Publishers, Enfield, 121–158.
- Arratia G, Gayet M (1995) Sensory canals and related bones of tertiary siluriform crania from Bolivia and North America and comparison with recent forms. Journal of Vertebrate Paleontology 15: 482–505. https://doi.org/10.1080/02724634.1995.10011243
- Arratia G, Huaquín L (1995) Morphology of the lateral line system and of the skin of diplomystids and certain primitive loricarioid catfishes and systematic and ecological considerations. Bonner Zoologische Monographien 36: 1–110.
- Arratia G, Menu-Marque S (1981) Revision of the freshwater catfishes of the genus Hatcheria (Siluriformes, Trichomycteridae) with commentaries on ecology and biogeography. Zoologischer Anzeiger 207: 88–111.
- Arratia G, Schultze HP (1990) The urohyal: development and homology within osteichthyans. Journal of Morphology 203: 247–282. https://doi.org/10.1002/jmor.1052030302
- Arratia G, Schultze HP (1991) Palatoquadrate and its ossifications: development and homology within osteichthyans. Journal of morphology 208: 1–81. https://doi.org/10.1002/jmor.1052080102
- Arratia G, Chang GA, Menu-Marque SM, Rojas MG (1978) About Bullockia gen. nov., Trichomycterus mendozensis n. sp. and revision of the family Trichomycteridae (Pisces, Siluriformes). Studies on Neotropical Fauna and Environment 13: 157–194. https://doi.org/10.1080/01650527809360539
- Baird RC (1971) The systematics, distribution, and zoogeography of the marine hatchetfishes (family Sternoptychidae). Bulletin of the Museum of Comparative Zoology 142: 1–522.
- Ballen GA, de Pinna MC (2021) A standardized terminology of spines in the order Siluriformes (Actinopterygii: Ostariophysi). Zoological Journal of the Linnean Society 194: 601–625. https://doi.org/10.1093/zoolinnean/zlab008
- Ballen GA, Pastana MN, Peixoto LA (2016) A new species of Farlowella (Siluriformes: Loricariidae) of the F. nattereri species-group from the rio Xingu basin, Mato Grosso, Brazil, with comments on Farlowella jauruensis, a poorly-known species from the upper rio Paraguai basin. Neotropical Ichthyology 14: 517–524. https://doi.org/10.1590/1982-0224-20160046
- Bamford TW (1948) Cranial development of Galeichthys felis. Proceedings of the Zoological Society of London 118: 364–391. https://doi.org/10.1111/j.1096-3642.1948.tb00383.x
- Barbieri LR, dos Santos RP, Andreata JV (1992) Reproductive biology of the marine catfish, Genidens genidens (Siluriformes, Ariidae), in the Jacarepaguá Lagoon system, Rio de Janeiro, Brazil. Environmental Biology of Fishes 35: 23–35. https://doi.org/10.1007/BF00001154
- de Beer GR (1937) The development of the vertebrate skull. Clarendon Press, Oxford, 552 pp.
- Bird NC, Mabee PM (2003) Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae). Developmental Dynamics 228: 337–357. https://doi.org/10.1002/dvdy.10387
- Birindelli JL, Sousa LM, Pérez MHS (2008) New species of thorny catfish, genus Leptodoras Boulenger (Siluriformes: Doradidae), from Tapajós and Xingu basins, Brazil. Neotropical Ichthyology 6: 465–480. https://doi.org/10.1590/S1679-62252008000300020
- Block AJ, Mabee PM (2012) Development of the mandibular, hyoid arch and gill arch skeleton in the Chinese barb Puntius semifasciolatus: comparisons of ossification sequences among Cypriniformes. Journal of Fish Biology 81: 54–80. https://doi.org/10.1111/j.1095-8649.2012.03307.x
- Bridge TW (1896) The mesial fins of ganoids and teleosts. Zoological Journal of the Linnean Society 25: 530–602. https://doi.org/10.1111/j.1096-3642.1896.tb00400.x
- Bridge TW, Haddon AC (1893) Contributions to the anatomy of fishes. II. The airbladder and Weberian ossicles in the siluroid fishes. Philosophical Transactions of the Royal Society of London B 184: 66–333. https://doi.org/10.1098/rspl.1889.0038
- Britz R, Conway KW (2009) Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae). Journal of Morphology 270: 389–412. https://doi.org/10.1002/jmor.10698
- Britz R, Conway KW (2016) Danionella dracula, an escape from the cypriniform Bauplan via developmental truncation?. Journal of morphology 277: 147–166. https://doi.org/10.1002/jmor.20486
- Britz R, Hoffmann M (2006) Ontogeny and homology of the claustra in otophysan Ostariophysi (Teleostei). Journal of Morphology 267: 909–923. https://doi.org/10.1002/jmor.10447
- Britz R, Johnson GD (2012) Ontogeny and homology of the skeletal elements that form the sucking disc of remoras (Teleostei, Echeneoidei, Echeneidae). Journal of Morphology 273: 1353–1366. https://doi.org/10.1002/jmor.20063
- Britz R, Kottelat M (2003) Descriptive osteology of the family Chaudhuriidae (Teleostei, Synbranchiformes, Mastacembeloidei), with a discussion of its relationships. American Museum Novitates 2003(3418): 1–62. https://doi.org/10.1206/0003-0082(2003)418<0001:DOOTFC>2.0.CO;2
- Britz R, Moritz T (2007) Reinvestigation of the osteology of the miniature African freshwater fishes Cromeria and Grasseichthys (Teleostei, Gonorynchiformes, Kneriidae), with comments on kneriid relationships. Zoosystematics and Evolution 83: 3–42. https://doi.org/10.1002/mmnz.200600016
- Britz R, Kakkassery F, Raghavan R (2014) Osteology of Kryptoglanis shajii, a stygobitic catfish (Teleostei: Siluriformes) from Peninsular India with a diagnosis of the new family Kryptoglanidae. Ichthyological Exploration of Freshwaters 24: 193–207.
- Brown BA, Ferraris CJ (1988) Comparative osteology of the Asian catfish family Chacidae: with the description of a new species from Burma. American Museum Novitates 1988(2907): 1–16.
- Carril J, Tambussi CP (2017) Skeletogenesis of Myiopsitta monachus (Psittaciformes) and sequence heterochronies in Aves. Evolution & Development 19: 17–28. https://doi.org/10.1111/ede.12211
- Calegari BB, Vari RP, Reis RE (2019) Phylogenetic systematics of the driftwood catfishes (Siluriformes: Auchenipteridae): a combined morphological and molecular analysis. Zoological Journal of the Linnean Society 187: 661–773. https://doi.org/10.1093/zoolinnean/zlz036
- Carvalho TP, Reis RE (2020) A New Miniature Species of Acanthobunocephalus (Silurifomes: Aspredinidae) from the Lower Purus River Basin, Amazon Basin, Brazil. Copeia 108: 347–357. https://doi.org/10.1643/CI-19-309
- Chranilov NS (1927) Beiträge zur Kenntnis des Weber’schen Apparates der Ostariophysi. 1. vergleichend-anatomische Übersicht der Knochenelemente des Weber’schen Apparates bei Cypriniformes. Zoologische Jahrbücher, Abteilung für Anatomie 49: 501–597.
- Coburn MM, Grubach PG (1998) Ontogeny of the Weberian apparatus in the armored catfish Corydoras paleatus (Siluriformes: Callichthyidae). Copeia (1998): 301–311. https://doi.org/10.2307/1447426
- Collinge WE (1895) On the sensory canal system of fishes. Teleostei – Suborder A. Physostomi. Proceedings of the Zoological Society of London 2: 274–298.
- Conway KW, Britz R (2007) Sexual dimorphism of the Weberian apparatus and pectoral girdle in Sundadanio axelrodi (Ostariophysi: Cyprinidae). Journal of Fish Biology 71: 1562–1570. https://doi.org/10.1111/j.1095-8649.2007.01646.x
- Conway KW, Kubicek KM, Britz R (2017) Morphological novelty and modest developmental truncation in Barboides, Africa’s smallest vertebrates (Teleostei: Cyprinidae). Journal of Morphology 278: 750–767. https://doi.org/10.1002/jmor.20670
- Conway KW, Kubicek KM, Britz R (2021) Extreme evolutionary shifts in developmental timing establish the miniature Danionella as a novel model in the neurosciences. Developmental Dynamics 250: 601–611. https://doi.org/10.1002/dvdy.280
- Coombs S, Janssen J, Webb JF (1988) Diversity of lateral line systems: evolutionary and functional considerations. In: Atema J, Fay RR, Pospper AN, Tavolga WN (Eds) Sensory biology of aquatic animals, Springer, New York, 553–593.
- Copp GH, Robert Britton J, Cucherousset J, García-Berthou E, Kirk R, Peeler E, Stakėnas S (2009) Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish and Fisheries 10: 252–282. https://doi.org/10.1111/j.1467-2979.2008.00321.x
- Cubbage CC, Mabee PM (1996) Development of the cranium and paired fins in the zebrafish Danio rerio (Ostariophysi, Cyprinidae). Journal of Morphology 229: 121–160. https://doi.org/10.1002/(SICI)1097-4687(199608)229:2<121::AID-JMOR1>3.0.CO;2-4
- Cumplido N, Allende ML, Arratia G (2020) From Devo to Evo: patterning, fusion and evolution of the zebrafish terminal vertebra. Frontiers in Zoology 17: 1–17. https://doi.org/10.1186/s12983-020-00364-y
- Diogo R (2004) Morphological Evolution, Aptations, Homoplasies, Constraints, and Evolutionary Trends: Catfishes as a Case Study on General Phylogeny and Macroevolution. Science Publishers, Enfield, 491 pp.
- Dutra GM, Peixoto LAW, Abrahão VP, Wosiacki WB, Menezes NA, de Santana CD (2021) Morphology-based phylogeny of Eigenmanniinae Mago-Leccia, 1978 (Teleostei: Gymnotiformes: Sternopygidae), with a new classification. Journal of Zoological Systematics and Evolutionary Research 59: 2010–2059. https://doi.org/10.1111/jzs.12535
- Egge JJD (2007) The osteology of the stonecat, Noturus flavus (Siluriformes: Ictaluridae), with comparisons to other siluriforms. Alabama Museum of Natural History Bulletin 25: 71–89.
- Fink SV, Fink WL (1981) Interrelationships of the ostariophysan fishes (Teleostei). Zoological Journal of the Linnean Society 72: 297–353. https://doi.org/10.1111/j.1096-3642.1981.tb01575.x
- Fink WL (1985) Phylogenetic interrelationships of the stomiid fishes (Teleostei: Stomiiformes). Miscellaneous Publications Museum of Zoology University of Michigan 171: 1–127.
- Fricke R, Eschmeyer WN, Fong JD (2022) Species by Family/Subfamily. (http://research.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp). Electronic version accessed 08 April 2022
- Friel JP, Lundberg JG (1996) Micromyzon akamai, gen. et sp. nov., a small and eyeless banjo catfish (Siluriformes: Aspredinidae) from the river channels of the lower Amazon basin. Copeia (1996): 641–648. https://doi.org/10.2307/1447528
- Fukushima M, Kohno H, Fujita K, Taki Y (1992) Ontogenetic development of the Weberian apparatus in the bitterling, Rhodeus ocellatus ocellatus. Journal of the Tokyo University of Fisheries 79: 195–200.
- Geerinckx T, Brunain M, Adriaens D (2007) Development of the osteocranium in the suckermouth armored catfish Ancistrus cf. triradiatus (Loricariidae, Siluriformes). Journal of Morphology 268: 254–274. https://doi.org/10.1002/jmor.10515
- Goswami A, Weisbecker V, Sánchez-Villagra MR (2009) Developmental modularity and the marsupial–placental dichotomy. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 312: 186–195. https://doi.org/10.1002/jez.b.21283
- Grande L, Lundberg JG (1988) Revision and redescription of the genus Astephus (Siluriformes: Ictaluridae) with a discussion of its phylogenetic relationships. Journal of Vertebrate Paleontology 8: 139–171. https://doi.org/10.1080/02724634.1988.10011694
- Grande T, Shardo JD (2002) Morphology and development of the postcranial skeleton in the channel catfish Ictalurus punctatus (Ostariophysi: Siluriformes). Field Museum of Natural History 1518: 1–30.
- Hardman M (2005) The phylogenetic relationships among non-diplomystid catfishes as inferred from mitochondrial cytochrome b sequences; the search for the ictalurid sister taxon (Otophysi: Siluriformes). Molecular phylogenetics and evolution 37: 700–720. https://doi.org/10.1016/j.ympev.2005.04.029
- Harrington SM, Harrison LB, Sheil CA (2013) Ossification sequence heterochrony among amphibians. Evolution & Development 15: 344–364. https://doi.org/10.1111/ede.12043
- Herrick CJ (1901) The cranial nerves and cutaneous sense organs of the North American silurid fishes. Journal of Comparative Neurology and Psychology 11: 177–249.
- Hilton EJ, Johnson GD (2007) When two equals three: developmental osteology and homology of the caudal skeleton in carangid fishes (Perciformes: Carangidae). Evolution & Development 9: 178–189. https://doi.org/10.1111/j.1525-142X.2007.00148.x
- Hoffmann M, Britz R (2006) Ontogeny and homology of the neural complex of otophysan Ostariophysi. Zoological Journal of the Linnean Society 147: 301–330. https://doi.org/10.1111/j.1096-3642.2006.00220.x
- Hubbs CL, Miller RR (1960) Potamarius, a new genus of ariid catfishes from the fresh waters of Middle America. Copeia (1960): 101–112. https://doi.org/10.2307/1440202
- Huysentruyt F, Adriaens D (2005) Descriptive osteology of Corydoras aeneus (Siluriformes: Callichthyidae). Cybium 29: 261–73.
- Huysentruyt F, Geerinckx T, Brunain M, Adriaens D (2011) Development of the osteocranium in Corydoras aeneus (Gill, 1858) Callichthyidae, Siluriformes. Journal of Morphology 272: 573–582. https://doi.org/10.1002/jmor.10935
- Ichiyanagi T, Kohno H, Fujita K (1997) Ontogenetic development of the Weberian ossicles in the bagrid catfish, Pseudobagrus ichikawai. Journal of the Tokyo University of Fisheries 84: 93–97.
- Jansen G, Devaere S, Weekers PHH, Adriaens D (2006) Phylogenetic relationships and divergence time estimate of African anguilliform catfish (Siluriformes: Clariidae) inferred from ribosomal gene and spacer sequences. Molecular Phylogenetics and Evolution 38: 65–78. https://doi.org/10.1016/j.ympev.2005.09.011
- Johnels AG (1957) The mode of terrestrial locomotion in Clarias. Oikos 8: 122–129. https://doi.org/10.2307/3564996
- Johnson GD (1983) Niphon spinosus: a primitive epinepheline serranid, with comments on the monophyly and intrarelationships of the Serranidae. Copeia (1983): 777–787. https://doi.org/10.2307/1444346
- Johnson GD, Washington BB (1987) Larvae of the Moorish idol, Zanclus cornutus, including a comparison with other larval acanthuroids. Bulletin of Marine Science 40: 494–511.
- Kaatz IM, Stewart DJ, Rice AN, Lobel PS (2010) Differences in pectoral fin spine morphology between vocal and silent clades of catfishes (order Siluriformes): ecomorphological implications. Current Zoology, 56: 73–89. https://doi.org/10.1093/czoolo/56.1.73
- Katano O, Saitoh K, Koizumi A (1988) Scatter-spawning of the catfish, Silurus asotus. Japanese Journal of Ichthyology 35: 203–211. https://doi.org/10.11369/jji1950.35.203
- Keyte AL, Smith KK (2010) Developmental origins of precocial forelimbs in marsupial neonates. Development 137: 4283–4294. https://doi.org/10.1242/dev.049445
- Kiernan JA (1990) Histological and histochemical methods: Theory and practice. Pergamon Press, New York, 571 pp.
- Kindred J (1919) The skull of Ameiurus. Illinois Biological Monograph, 5: 1–120.
- Klein A, Münz H, Bleckmann H (2013) The functional significance of lateral line canal morphology on the trunk of the marine teleost Xiphister atropurpureus (Stichaeidae). Journal of comparative physiology A 199: 735–749. https://doi.org/10.1007/s00359-013-0834-6
- Kubicek KM, Conway KW (2016) Developmental osteology of Sciaenops ocellatus and Cynoscion nebulosus (Teleostei: Sciaenidae), economically important sciaenids from the western Atlantic. Acta Zoologica 97: 267–301. https://doi.org/10.1111/azo.12122
- Kubicek KM, Britz R, Conway KW (2019) Ontogeny of the catfish pectoral-fin spine (Teleostei: Siluriformes). Journal of Morphology 280: 339–359. https://doi.org/10.1002/jmor.20947
- Lundberg JG (1975) Homologies of the upper shoulder girdle and temporal region bones in catfishes (Order Siluriformes), with comments on the skull of the Helogeneidae. Copeia (1975), 66–74. https://doi.org/10.2307/1442407
- Lundberg JG (1975) The fossil catfishes of North America. University of Michigan Museum of Paleontology, Papers on Paleontology 11: 1–51.
- Lundberg JG (1982) The comparative anatomy of the toothless blindcat, Trogloglanis pattersoni Eigenmann, with a phylogenetic analysis of the ictalurid catfishes. Miscellaneous Publications of the Museum of Zoology University Michigan 163: 1–85.
- Lundberg JG, Baskin JN (1969) The caudal skeleton of the catfishes, order Siluriformes. American Museum Novitates 1969(2398): 1–49.
- Lundberg JG, Mago-Leccia F (1986) A review of Rhabdolichops (Gymnotiformes, Sternopygidae), a genus of South American freshwater fishes, with descriptions of four new species. Proceedings of the Academy of Natural Sciences of Philadelphia 138: 53–85.
- Lundberg JG, Hendrickson DA, Luckenbill KR, Mariangeles AH (2017) Satan’s skeleton revealed: a tomographic and comparative osteology of Satan eurystomus, the subterranean Widemouth Blindcat (Siluriformes, Ictaluridae). Proceedings of the Academy of Natural Sciences of Philadelphia 165: 117–173. https://doi.org/10.1635/053.165.0108
- Lundberg JG, Sullivan JP, Rodiles-Hernández R, Hendrickson DA (2007) Discovery of African roots for the Mesoamerican Chiapas catfish, Lacantunia enigmatica, requires an ancient intercontinental passage. Proceedings of the Academy of Natural Sciences of Philadelphia 156: 39–53. https://doi.org/10.1635/0097-3157(2007)156[39:DOARFT]2.0.CO;2
- Mabee PM, Trendler TA (1996) Development of the cranium and paired fins in Betta splendens (Teleostei: Percomorpha): intraspecific variation and interspecific comparisons. Journal of Morphology 227: 249–287. https://doi.org/10.1002/(SICI)1097-4687(199603)227:3<249::AID-JMOR1>3.0.CO;2-1
- Maehata M (2007) Reproductive ecology of the Far Eastern catfish, Silurus asotus (Siluridae), with a comparison to its two congeners in Lake Biwa, Japan. Environmental Biology of Fishes, 78: 135–146. https://doi.org/10.1007/s10641-006-9083-7
- Mayden RL, Burr BM, Dewey SL (1980) Aspects of the life history of the Ozark madtom, Noturus albater, in southeastern Missouri (Pisces: Ictaluridae). American Midland Naturalist 104: 335–340. https://doi.org/10.2307/2424874
- Mattox GM, Britz R, Toledo-Piza M (2014) Skeletal development and ossification sequence of the characiform Salminus brasiliensis (Ostariophysi: Characidae). Ichthyological Exploration of Freshwaters, 25: 103–158.
- Mattox GM, Britz R, Toledo-Piza M (2016) Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). Journal of Morphology, 277: 65–85. https://doi.org/10.1002/jmor.20477
- McMurrich JP (1884) On the osteology of Amiurus catus (L.) gill. Zoologisches Anzeiger 168: 296–299.
- Mistri A, Kumari U, Mittal S, Mittal AK (2018) Morphological specializations of the epidermis of an angler catfish Chaca chaca (Siluriformes, Chacidae) in relation to its ecological niche: A scanning electron microscopic investigation. Microscopy research and technique 81: 439–448. https://doi.org/10.1002/jemt.22996
- Nakae M, Asaoka R, Wada H, Sasaki K (2012) Fluorescent dye staining of neuromasts in live fishes: an aid to systematic studies. Ichthyological research, 59: 286–290. https://doi.org/10.1007/s10228-012-0274-2
- Nakae M, Kuroki M, Sato M, Sasaki K (2021) The lateral line system and its innervation in the Japanese eel Anguilla japonica (Teleostei: Elopomorpha: Anguillidae). Journal of Morphology, 282: 863–873. https://doi.org/10.1002/jmor.21353
- Nelson JS, Grande TC, Wilson MV (2016) Fishes of the World (5th ed.). John Wiley & Sons, Hoboken, 752 pp.
- Patterson C (1977) Cartilage bones, dermal bones and membrane bones, or the exoskeleton versus the endoskeleton. In: Andrews SM, Miles RS, Walker AD (Eds) Problems in Vertebrate Evolution. London: Academic Press, 77–121.
- Patterson C, Johnson GD (1995) The intermuscular bones and ligaments of teleostean fishes. Smithsonian Contributions to Zoology 559: 1–85.
- Paxton CGM (1997) Shoaling and activity levels in Corydoras. Journal of Fish Biology 51: 496–502. https://doi.org/10.1111/j.1095-8649.1997.tb01507.x
- Pinion AK, Siegel DS, Britz R, Martínez-García R, Álvarez-González CA, Conway KW (2021) The larval attachment organ of the tropical gar Atractosteus tropicus Gill, 1863 (Lepisosteiformes: Lepisosteidae). Journal of Fish Biology 99: 418–424. https://doi.org/10.1111/jfb.14733
- de Pinna MCC, Ng HH (2004) The second ural centrum in Siluriformes and its implication for the monophyly of superfamily Sisoroidea (Teleostei, Ostariophysi). American Museum Novitates, 2004: 1–23. https://doi.org/10.1206/0003-0082(2004)437<0001:TSUCIS>2.0.CO;2
- de Pinna MCC, Winemiller KO (2000) A new species of Ammoglanis (Siluriformes: Trichomycteridae) from Venezuela. Ichthyological Exploration of Freshwaters 11: 255–264.
- de Pinna MCC, Ferraris Jr CJ, Vari RP (2007) A phylogenetic study of the Neotropical catfish family Cetopsidae (Osteichthyes, Ostariophysi, Siluriformes), with a new classification. Zoological Journal of the Linnean Society 150: 755–813. https://doi.org/10.1111/j.1096-3642.2007.00306.x
- de Pinna MCC, Reis V, Britski H (2020) A new species of Trichogenes (Siluriformes, Trichomycteridae), with a discussion on the homologies of the anterior orbital bones in trichomycterids and other loricarioids. American Museum Novitates 2020(3951): 1–27. https://doi.org/10.1206/3951.1
- Rao KS, Lakshmi K (1984) Head skeleton of the marine catfish Arius tenuispinis Day (Osteichthyes: Siluriformes, Ariidae). Journal of Morphology 181: 221–238. https://doi.org/10.1002/jmor.1051810208
- Reed HD (1924) The morphology and growth of the spines of siluroid fishes. Journal of Morphology 38: 431–451. https://doi.org/10.1002/jmor.1050380306
- Regan CT (1911) LXV.—The classification of the Teleostean fishes of the order Ostariophysi.—2. Siluroidea. Journal of Natural History 8: 553–577. https://doi.org/10.1080/00222931108693067
- Reiss JO (1989) The meaning of developmental time: a metric for comparative embryology. The American Naturalist 134: 170–189. https://doi.org/10.1086/284974
- Reynolds JD (1971) Biology of the small pelagic fishes in the New Volta Lake in Ghana: I. schooling and migrations. Hydrobiologia 38: 79–91. https://doi.org/10.1007/BF00036795
- Rodiles-Hernández R, Hendrickson DA, Lundberg JG, Humphries JM (2005) Lacantunia enigmatica (Teleostei: Siluriformes) a new and phylogenetically puzzling freshwater fish from Mesoamerica. Zootaxa 1000: 1–24. https://doi.org/10.11646/zootaxa.1000.1.1
- Sabaj MH (2020) Codes for natural history collections in ichthyology and herpetology. Copeia 108: 593–669. https://doi.org/10.1643/ASIHCODONS2020
- Sánchez-Villagra MR, Goswami A, Weisbecker V, Mock O, Kuratani S (2008) Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evolution & Development 10(5): 519–530. https://doi.org/10.1111/j.1525-142X.2008.00267.x
- Sato M, Asaoka R, Nakae M, Sasaki K (2017) The lateral line system and its innervation in Lateolabrax japonicus (Percoidei incertae sedis) and two apogonids (Apogonidae), with special reference to superficial neuromasts (Teleostei: Percomorpha). Ichthyological Research, 64: 308–330. https://doi.org/10.1007/s10228-016-0568-x
- Schultze HP, Arratia G (2013) The caudal skeleton of basal teleosts, its conventions, and some of its major evolutionary novelties in a temporal dimension. Mesozoic Fishes 5: 187–246.
- Slobodian V, Pastana MN (2018) Description of a new Pimelodella (Siluriformes: Heptapteridae) species with a discussion on the upper pectoral girdle homology of Siluriformes. Journal of Fish Biology 93: 901–916. https://doi.org/10.1111/jfb.13795
- Sørensen W (1890) Om Forbeninger i Svømmeblaeren, Pleura og Aortas Vaeg og Sammelsmeltning deraf med Hvirvelsøjlen saerlig hos Siluroiderne, samt de saakaldte Weberske Knoglers Morfologi. Det Kongelike Danske Videnskabernes Selskabs Skrifter, 6 Raekke, Naturvidenskabelig og Matematisk Afdeling VI 2: 1–88.
- Srinivasachar HR (1958) Development of the skull in catfishes. V. Development of skull in Heteropneustes fossilis (Bloch). Proceedings of the National Institute of Sciences India 24B: 165–190.
- Starks EC (1930) The primary shoulder girdle of the bony fishes. Stanford University Publications, University Series, Biological Sciences 6: 147–239.
- Sumi K, Asaoka R, Nakae M, Sasaki K (2015) Innervation of the lateral line system in the blind cavefish Astyanax mexicanus (Characidae) and comparisons with the eyed surface-dwelling form. Ichthyological Research 62: 420–430. https://doi.org/10.1007/s10228-015-0458-7
- Tavolga WN (1962) Mechanisms of sound production in the ariid catfishes Galeichthys and Bagre. Bulletin of the American Museum of Natural History 124: 1–30. http://hdl.handle.net/2246/1211
- Taylor WR (1967) An enzyme method of clearing and staining small vertebrates. Proceedings of the United States National Museum 122: 1–17.
- Taylor WR (1969) A revision of the catfish genus Noturus Rafinesque with an analysis of higher groups in the Ictaluridae. Bulletin of the United States National Museum 282: 1–315.
- Taylor WR, Van Dyke GC (1985) Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 9: 107–119.
- Tencatt LFC, Ohara WM (2016) Two new species of Corydoras Lacépède, 1803 (Siluriformes: Callichthyidae) from the rio Madeira basin, Brazil. Neotropical Ichthyology 14(1): 139–154. https://doi.org/10.1590/1982-0224-20150063
- Tyler JC (1980) Osteology, phylogeny, and higher classification of the fishes of the order Plectognathi (Tetraodontiformes). NOAA (National Oceanic and Atmospheric Administration) Technical Report NMFS (National Marine Fisheries Service) Circular 434: 1–422.
- Vanscoy T, Lundberg JG, Luckenbill KR (2015) Bony ornamentation of the catfish pectoral-fin spine: comparative and developmental anatomy, with an example of fin-spine diversity using the Tribe Brachyplatystomini (Siluriformes, Pimelodidae). Proceedings of the Academy of Natural Sciences of Philadelphia 164: 177–212. https://doi.org/10.1635/053.164.0107
- Vigliotta TR (2008) A phylogenetic study of the African catfish family Mochokidae (Osteichthyes, Ostariophysi, Siluriformes), with a key to genera. Proceedings of the Academy of Natural Sciences of Philadelphia 157: 73–136. https://doi.org/10.1635/0097-3157(2008)157[73:APSOTA]2.0.CO;2
- Walker MB, Kimmel CB (2007) A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotechnic & Histochemistry 82: 23–28. https://doi.org/10.1080/10520290701333558
- Webb JF, Shirey JE (2003) Postembryonic development of the cranial lateral line canals and neuromasts in zebrafish. Developmental Dynamics 228: 370–385. https://doi.org/10.1002/dvdy.10385
- Weitzman SH (1962) The osteology of Brycon meeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyological Bulletin 8: 1–77.
- Werneburg I, Sánchez-Villagra MR (2015) Skeletal heterochrony is associated with the anatomical specializations of snakes among squamate reptiles. Evolution 69: 254–263. https://doi.org/10.1111/evo.12559